A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulae.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of cations and anions. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−) or polyatomic, such as sulfate.
Toluene, also known as toluol, is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH₃) attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, strong-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested.
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about 1 million metric tons (980,000 long tons; 1,100,000 short tons) in 1985.
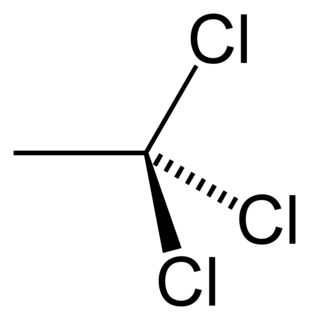
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.

The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

Iron(III) chloride is the inorganic compound with the formula. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine that has an effect on the chemical behavior of the molecule. The chloroalkane class provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names and applications. Organochlorides are very useful compounds in many applications, but some are of profound environmental concern.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.
2,2,2-Trichloroethanol is the chemical compound with formula Cl
3C−CH
2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms at position 2 replaced by chlorine atoms. It is a clear flammable liquid at room temperature, colorless when pure but often with a light yellow color.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original cling-wrap, Saran, for food, but this application has been phased out.
1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula C2H3Cl3. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane.

Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity. Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The reaction is driven by the loss of volatile TiCl4:
Dehydrohalogenation is an elimination reaction that eliminates (removes) a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Isobutyl chloride (1-chloro-2-methylpropane) is a compound of chlorine, carbon, and hydrogen. It is a chlorinated derivative of isobutane.
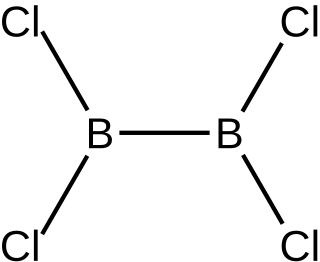
Diboron tetrachloride is a chemical compound with the formula B2Cl4. It is a colorless liquid. The electrical discharge procedure of boron trichloride can be divided into three steps:
In chemistry, vinylidenes are compounds with the functional group C=CH2. An example is 1,1-dichloroethene (CCl2=CH2) commonly called vinylidene chloride. It and vinylidene fluoride are precursors to commercially useful polymers.

Dichloroacetyl chloride is the organic compound with the formula CHCl2COCl. It is the acyl chloride of dichloroacetic acid. It is a colourless liquid and is used in acylation reactions.






