In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.
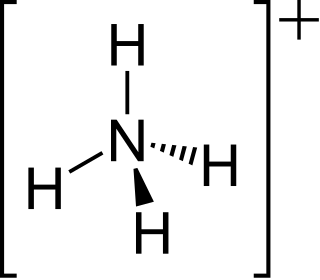
The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.
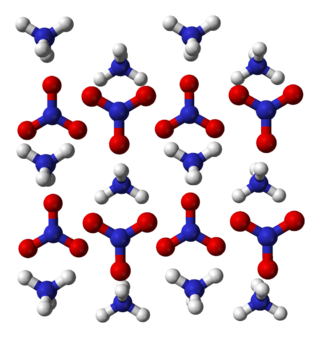
Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Global production was estimated at 21.6 million tonnes in 2017.
Benzonitrile is the chemical compound with the formula C6H5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.

Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to groups or a class of compounds with the same ring structure as the parent compound. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.

Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.

From 1948 to 1975, the U.S. Army Chemical Corps conducted classified human subject research at the Edgewood Arsenal facility in Maryland. The purpose was to evaluate the impact of low-dose chemical warfare agents on military personnel and to test protective clothing, pharmaceuticals, and vaccines. A small portion of these studies were directed at psychochemical warfare and grouped under the prosaic title of the "Medical Research Volunteer Program" (1956–1975). The MRVP was also driven by intelligence requirements and the need for new and more effective interrogation techniques.

2-Naphthylamine is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen, chalcogen, or halogen. The oldest-known onium ion, and the namesake for the class, is ammonium, NH+4, the protonated derivative of ammonia, NH3.
The Leuckart reaction is the chemical reaction that converts aldehydes or ketones to amines by reductive amination in the presence of heat. The reaction, named after Rudolf Leuckart, uses either ammonium formate or formamide as the nitrogen donor and reducing agent. It requires high temperatures, usually between 120 and 130 °C; for the formamide variant, the temperature can be greater than 165 °C.
The chemical substance 1,2-dioxetane is a heterocyclic, organic compound with formula C2O2H4, containing a ring of two adjacent oxygen atoms and two adjacent carbon atoms. It is therefore an organic peroxide, and can be viewed as a dimer of formaldehyde.

Ammonium bisulfate, also known as ammonium hydrogen sulfate, is a white, crystalline solid with the formula (NH4)HSO4. This salt is the product of the half-neutralization of sulfuric acid by ammonia.
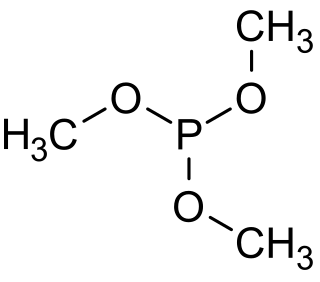
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
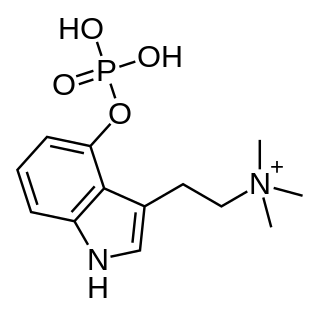
Aeruginascin or N,N,N-trimethyl-4-phosphoryloxytryptamine is an indoleamine derivative which occurs naturally within the mushrooms Inocybe aeruginascens and Pholiotina cyanopus, and Psilocybe cubensis. Aeruginascin is the N-trimethyl analogue of psilocybin. It is closely related to the frog skin toxin bufotenidine (5-HTQ), a potent 5-HT3 receptor agonist, but the aeruginascin metabolite 4-HO-TMT shows strong binding at the 5-HT2 receptors similar to psilocin. The first scientific literature about the pharmacological effects of aeruginascin is from a study published by Gartz in 1989. Across 23 analyzed cases of accidental hallucinogenic mushroom poisonings, people who had ingested the mushroom Inocybe aeruginascens reported only euphoric experiences. This is in contrast to the slight and in some cases extremely dysphoric experiences reported from the accidental ingestion of non aeruginascin containing mushrooms (containing solely psilocybin and psilocin).
Furfurylamine is an aromatic amine typically formed by the reductive amination of furfural with ammonia.

Nitrogen pentahydride, also known as ammonium hydride is a hypothetical compound with the chemical formula NH5. There are two theoretical structures of nitrogen pentahydride. One structure is trigonal bipyramidal molecular geometry type NH5 molecule. Its nitrogen atom and hydrogen atoms are covalently bounded, and its symmetry group is D3h. Another predicted structure of nitrogen pentahydride is an ionic compound, make up of an ammonium ion and a hydride ion (NH4+H−). Until now, no one has synthesized this substance, or proved its existence, and related experiments have not directly observed nitrogen pentahydride. It is only speculated that it may be a reactive intermediate based on reaction products. Theoretical calculations show this molecule is thermodynamically unstable. The reason might be similar to the instability of nitrogen pentafluoride, so the possibility of its existence is low. However, nitrogen pentahydride might exist in special conditions or high pressure. Nitrogen pentahydride was considered for use as a solid rocket fuel for research in 1966.
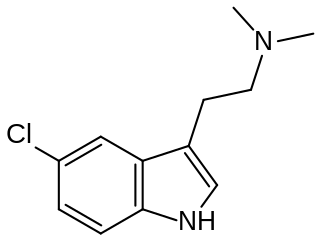
5-Chloro-N,N-dimethyltryptamine (5-chloro-DMT) is a tryptamine derivative related to compounds such as 5-bromo-DMT and 5-fluoro-DMT. It acts as a serotonin receptor agonist and has primarily sedative effects in animal studies. It has been sold as a designer drug.















