Related Research Articles

Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At 20 °C (68 °F), the speed of sound in air is about 343 metres per second, or one kilometre in 2.91 s or one mile in 4.69 s. It depends strongly on temperature as well as the medium through which a sound wave is propagating. At 0 °C (32 °F), the speed of sound in air is about 331 m/s.
In physics and materials science, elasticity is the ability of a body to resist a distorting influence and to return to its original size and shape when that influence or force is removed. Solid objects will deform when adequate loads are applied to them; if the material is elastic, the object will return to its initial shape and size after removal. This is in contrast to plasticity, in which the object fails to do so and instead remains in its deformed state.

In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to slide over each other at low stress levels and is known as glide or slip. The crystalline order is restored on either side of a glide dislocation but the atoms on one side have moved by one position. The crystalline order is not fully restored with a partial dislocation. A dislocation defines the boundary between slipped and unslipped regions of material and as a result, must either form a complete loop, intersect other dislocations or defects, or extend to the edges of the crystal. A dislocation can be characterised by the distance and direction of movement it causes to atoms which is defined by the Burgers vector. Plastic deformation of a material occurs by the creation and movement of many dislocations. The number and arrangement of dislocations influences many of the properties of materials.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.
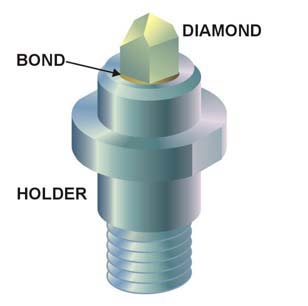
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

The mechanical properties of carbon nanotubes reveal them as one of the strongest materials in nature. Carbon nanotubes (CNTs) are long hollow cylinders of graphene. Although graphene sheets have 2D symmetry, carbon nanotubes by geometry have different properties in axial and radial directions. It has been shown that CNTs are very strong in the axial direction. Young's modulus on the order of 270 - 950 GPa and tensile strength of 11 - 63 GPa were obtained.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.
Carbon nanotube springs are springs made of carbon nanotubes (CNTs). They are an alternate form of high density, lightweight, reversible energy storage based on the elastic deformations of CNTs. Many previous studies on the mechanical properties of CNTs have revealed that they possess high stiffness, strength and flexibility. The Young's modulus of CNTs is 1 TPa and they have the ability to sustain reversible tensile strains of 6% and the mechanical springs based on these structures are likely to surpass the current energy storage capabilities of existing steel springs and provide a viable alternative to electrochemical batteries. The obtainable energy density is predicted to be highest under tensile loading, with an energy density in the springs themselves about 2500 times greater than the energy density that can be reached in steel springs, and 10 times greater than the energy density of lithium-ion batteries.
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials.

A metallic microlattice is a synthetic porous metallic material consisting of an ultra-light metal foam. With a density as low as 0.99 mg/cm3 (0.00561 lb/ft3), it is one of the lightest structural materials known to science. It was developed by a team of scientists from California-based HRL Laboratories, in collaboration with researchers at University of California, Irvine and Caltech, and was first announced in November 2011. The prototype samples were made from a nickel-phosphorus alloy. In 2012, the microlattice prototype was declared one of 10 World-Changing Innovations by Popular Mechanics. Metallic microlattice technology has numerous potential applications in automotive and aeronautical engineering. A detailed comparative review study among other types of metallic lattice structures showed them to be beneficial for light-weighting purposes but expensive to manufacture.

Aerographite is a synthetic foam consisting of a porous interconnected network of tubular carbon. With a density of 180 g/m3 it is one of the lightest structural materials ever created. It was developed jointly by a team of researchers at the University of Kiel and the Technical University of Hamburg in Germany, and was first reported in a scientific journal in June 2012.
Aerographene or graphene aerogel is, as of April 2020, the least dense solid known, at 160 g/m3, less than helium. It is approximately 7.5 times less dense than air. Note that the cited density does not include the weight of the air incorporated in the structure: it does not float in air. It was developed at Zhejiang University. The material reportedly can be produced at the scale of cubic meters.
Reversibly assembled cellular composite materials (RCCM) are three-dimensional lattices of modular structures that can be partially disassembled to enable repairs or other modifications. Each cell incorporates structural material and a reversible interlock, allowing lattices of arbitrary size and shape. RCCM display three-dimensional symmetry derived from the geometry as linked.
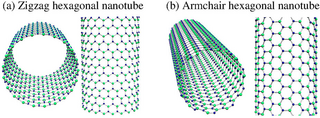
Gallium nitride nanotubes (GaNNTs) are nanotubes of gallium nitride. They can be grown by chemical vapour deposition.
Microscale structural metamaterials are synthetic structures that are aimed to yield specific desired mechanical advantages. These designs are often inspired by natural cellular materials such as plant and bone tissue which have superior mechanical efficiency due to their low weight to stiffness ratios.
A graphene morphology is any of the structures related to, and formed from, single sheets of graphene. 'Graphene' is typically used to refer to the crystalline monolayer of the naturally occurring material graphite. Due to quantum confinement of electrons within the material at these low dimensions, small differences in graphene morphology can greatly impact the physical and chemical properties of these materials. Commonly studied graphene morphologies include the monolayer sheets, bilayer sheets, graphene nanoribbons and other 3D structures formed from stacking of the monolayer sheets.
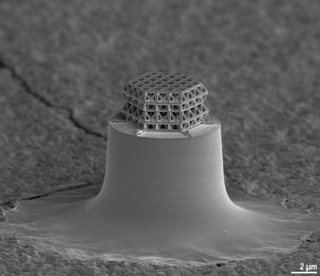
A nanolattice is a synthetic porous material consisting of nanometer-size members patterned into an ordered lattice structure, like a space frame. The nanolattice is a newly emerged material class that has been rapidly developed over the last decade. Nanolattices redefine the limits of the material property space. Despite being composed of 50-99% of air, nanolattices are very mechanically robust because they take advantage of size-dependent properties that we generally see in nanoparticles, nanowires, and thin films. The most typical mechanical properties of nanolattices include ultrahigh strength, damage tolerance, and high stiffness. Thus, nanolattices have a wide range of applications.
References
- ↑ "Designing amazingly strong materials from the bottom up". KurzweilAI. Retrieved 2013-09-13.
- 1 2 Jang, D.; Meza, L. R.; Greer, F.; Greer, J. R. (2013). "Fabrication and deformation of three-dimensional hollow ceramic nanostructures" (PDF). Nature Materials. 12 (10): 893–898. Bibcode:2013NatMa..12..893J. doi:10.1038/nmat3738. PMID 23995324.
- ↑ Shankland, Stephen (2011-11-18). "Breakthrough material is barely more than air | Deep Tech - CNET News". News.cnet.com. Retrieved 2013-09-13.
- ↑ Schaedler, T. A.; Jacobsen, A. J.; Torrents, A.; Sorensen, A. E.; Lian, J.; Greer, J. R.; Valdevit, L.; Carter, W. B. (2011). "Ultralight Metallic Microlattices". Science. 334 (6058): 962–965. Bibcode:2011Sci...334..962S. doi:10.1126/science.1211649. PMID 22096194. S2CID 23893516.
- ↑ Silva, J. M.; Duarte, A. R. C.; Custódio, C. A.; Sher, P.; Neto, A. I.; Pinho, A. N. C. M.; Fonseca, J.; Reis, R. L.; Mano, J. O. F. (2013). "Nanostructured Hollow Tubes Based on Chitosan and Alginate Multilayers". Advanced Healthcare Materials. 3 (3): 433–440. doi:10.1002/adhm.201300265. hdl: 1822/25593 . PMID 23983205. S2CID 33369579.