Related Research Articles

Corticotropin-releasing hormone (CRH) is a peptide hormone involved in stress responses. It is a releasing hormone that belongs to corticotropin-releasing factor family. In humans, it is encoded by the CRH gene. Its main function is the stimulation of the pituitary synthesis of adrenocorticotropic hormone (ACTH), as part of the hypothalamic–pituitary–adrenal axis.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.
Corticotropin-releasing factor family, CRF family is a family of related neuropeptides in vertebrates. This family includes corticotropin-releasing hormone, urotensin-I, urocortin, and sauvagine. The family can be grouped into 2 separate paralogous lineages, with urotensin-I, urocortin and sauvagine in one group and CRH forming the other group. Urocortin and sauvagine appear to represent orthologues of fish urotensin-I in mammals and amphibians, respectively. The peptides have a variety of physiological effects on stress and anxiety, vasoregulation, thermoregulation, growth and metabolism, metamorphosis and reproduction in various species, and are all released as prohormones.
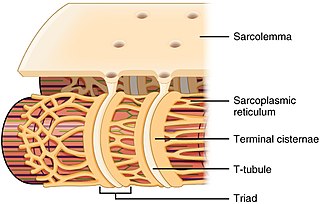
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger. Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Atrial natriuretic peptide (ANP) or atrial natriuretic factor (ANF) is a natriuretic peptide hormone secreted from the cardiac atria that in humans is encoded by the NPPA gene. Natriuretic peptides are a family of hormone/paracrine factors that are structurally related. The main function of ANP is causing a reduction in expanded extracellular fluid (ECF) volume by increasing renal sodium excretion. ANP is synthesized and secreted by cardiac muscle cells in the walls of the atria in the heart. These cells contain volume receptors which respond to increased stretching of the atrial wall due to increased atrial blood volume.

Vasodilation is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Milrinone, sold under the brand name Primacor, is a pulmonary vasodilator used in patients who have heart failure. It is a phosphodiesterase 3 inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Milrinone also works to vasodilate which helps alleviate increased pressures (afterload) on the heart, thus improving its pumping action. While it has been used in people with heart failure for many years, studies suggest that milrinone may exhibit some negative side effects that have caused some debate about its use clinically.

Urocortin is a protein that in humans is encoded by the UCN gene. Urocortin belongs to the corticotropin-releasing factor (CRF) family of proteins which includes CRF, urotensin I, sauvagine, urocortin II and urocortin III. Urocortin is involved in the mammalian stress response, and regulates aspects of appetite and stress response.

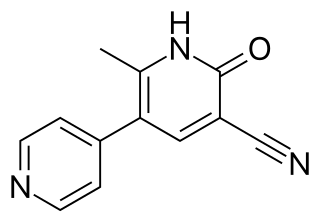
Amrinone, also known as inamrinone, and sold as Inocor, is a pyridine phosphodiesterase 3 inhibitor. It is a drug that may improve the prognosis in patients with congestive heart failure. Amrinone has been shown to increase the contractions initiated in the heart by high-gain calcium induced calcium release (CICR). The positive inotropic effect of amrinone is mediated by the selective enhancement of high-gain CICR, which contributes to the contraction of myocytes by phosphorylation through cAMP dependent protein kinase A (PKA) and Ca2+ calmodulin kinase pathways.
Neuromedin U is a neuropeptide found in the brain of humans and other mammals, which has a number of diverse functions including contraction of smooth muscle, regulation of blood pressure, pain perception, appetite, bone growth, and hormone release. It was first isolated from the spinal cord in 1985, and named after its ability to cause smooth muscle contraction in the uterus.

Corticotropin-releasing hormone receptor 1 (CRHR1) is a protein, also known as CRF1, with the latter (CRF1) now being the IUPHAR-recommended name. In humans, CRF1 is encoded by the CRHR1 gene at region 17q21.31, beside micrototubule-associated protein tau MAPT.

Corticotropin-releasing hormone receptor 2 (CRHR2) is a protein, also known by the IUPHAR-recommended name CRF2, that is encoded by the CRHR2 gene and occurs on the surfaces of some mammalian cells. CRF2 receptors are type 2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH, a peptide of 41 amino acids synthesized in the hypothalamus, is the principal neuroregulator of the hypothalamic-pituitary-adrenal axis, signaling via guanine nucleotide-binding proteins (G proteins) and downstream effectors such as adenylate cyclase. The CRF2 receptor is a multi-pass membrane protein with a transmembrane domain composed of seven helices arranged in a V-shape. CRF2 receptors are activated by two structurally similar peptides, urocortin II, and urocortin III, as well as CRH.

Corticotropin-releasing factor-binding protein is a protein that in humans is encoded by the CRHBP gene. It belongs to corticotropin-releasing hormone binding protein family.

Urocortin-2 is a protein that in humans is encoded by the UCN2 gene.

Urocortin-3 is a protein that in humans is encoded by the UCN3 gene. It belongs to the corticotropin-releasing hormone family.

Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.
A Corticotropin-releasing hormone antagonist is a specific type of receptor antagonist that blocks the receptor sites for corticotropin-releasing hormone, also known as corticotropin-releasing factor (CRF), which synchronizes the behavioral, endocrine, autonomic, and immune responses to stress by controlling the hypothalamic-pituitary-adrenal axis. CRH antagonists thereby block the consequent secretions of ACTH and cortisol due to stress, among other effects.

Verucerfont (GSK-561,679) is a drug developed by GlaxoSmithKline which acts as a CRF-1 antagonist. Corticotropin releasing factor (CRF), also known as Corticotropin releasing hormone, is an endogenous peptide hormone which is released in response to various triggers such as chronic stress, and activates the two corticotropin-releasing hormone receptors CRH-1 and CRH-2. This then triggers the release of corticotropin (ACTH), another hormone which is involved in the physiological response to stress.
Sauvagine is a neuropeptide from the corticotropin-releasing factor (CRF) family of peptides and is orthologous to the mammalian hormone, urocortin 1, and the teleost fish hormone, urotensin 1. It is 40 amino acids in length, and has the sequence XGPPISIDLSLELLRKMIEIEKQEKEKQQAANNRLLLDTI-NH2, with a pyrrolidone carboxylic acid modification at the N-terminal and amidation of the C-terminal isoleucine residue. It was originally isolated from the skin of the frog Phyllomedusa sauvagii. Given its relation to other CRF-related peptides, it exerts similar physiological effects as corticotropin-releasing hormone.
References
- 1 2 3 4 5 Smani T, Calderon E, Rodriguez-Moyano M, Dominguez-Rodriguez A, Diaz I, Ordóñez A (2011). "Urocortin-2 induces vasorelaxation of coronary arteries isolated from patients with heart failure". Clinical and Experimental Pharmacology & Physiology. 38 (1): 71–6. doi:10.1111/j.1440-1681.2010.05466.x. PMID 21105894. S2CID 34132312.
- 1 2 3 4 5 Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K (2000). "Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart" (PDF). American Journal of Physiology. Heart and Circulatory Physiology. 279 (6): H3031–9. doi:10.1152/ajpheart.2000.279.6.h3031. PMID 11087261. S2CID 14876819.
- 1 2 Kumar V, Abbas AK, Fausto N, Mitchell RN, Burns D (2007). "From The Heart". In Kumar V, Abbas AK, Fausto N, Mitchell RN (eds.). Robbins Basic Pathology (8th ed.). Philadelphia PA: Saunders. pp. 379–419.
- 1 2 Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards M (2007). "Urocortin 2 infusion in human heart failure". European Heart Journal. 28 (21): 2589–97. doi: 10.1093/eurheartj/ehm340 . PMID 17720993.
- 1 2 Meili-Butz S, Bühler K, John D, Buser P, Vale WW, Peterson KL, Brink M, Dieterle T (2010). "Acute effects of urocortin 2 on cardiac function and propensity for arrhythmias in an animal model of hypertension-induced left ventricular hypertrophy and heart failure". European Journal of Heart Failure. 12 (8): 797–804. doi: 10.1093/eurjhf/hfq054 . PMID 20388649.
- 1 2 Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL (2004). "The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure". Proceedings of the National Academy of Sciences of the United States of America. 101 (10): 3697–702. Bibcode:2004PNAS..101.3697B. doi: 10.1073/pnas.0307324101 . PMC 373525 . PMID 14990799.
- 1 2 3 4 Yang LZ, Kockskämper J, Khan S, Suarez J, Walther S, Doleschal B, Unterer G, Khafaga M, Mächler H, Heinzel FR, Dillmann WH, Pieske B, Spiess J (January 2011). "cAMP- and Ca²(+) /calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes". British Journal of Pharmacology. 162 (2): 544–56. doi:10.1111/j.1476-5381.2010.01067.x. PMC 3031072 . PMID 20942811.
- 1 2 3 4 Yang LZ, Tovote P, Rayner M, Kockskämper J, Pieske B, Spiess J (April 2010). "Corticotropin-releasing factor receptors and urocortins, links between the brain and the heart". European Journal of Pharmacology. 632 (1–3): 1–6. doi:10.1016/j.ejphar.2010.01.027. PMID 20132811.
- 1 2 3 4 5 Yang LZ, Kockskämper J, Heinzel FR, Hauber M, Walther S, Spiess J, Pieske B (February 2006). "Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A". Cardiovascular Research. 69 (2): 402–11. doi: 10.1016/j.cardiores.2005.10.015 . PMID 16386238.
- ↑ Smani T, Domínguez-Rodríguez A, Hmadcha A, Calderón-Sánchez E, Horrillo-Ledesma A, Ordóñez A (2007). "Role of Ca2+-independent phospholipase A2 and store-operated pathway in urocortin-induced vasodilatation of rat coronary artery". Circulation Research. 101 (11): 1194–203. doi: 10.1161/CIRCRESAHA.107.159053 . PMID 17885217.