
Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating the body's adaptive immunity, they help prevent sickness from an infectious disease. When a sufficiently large percentage of a population has been vaccinated, herd immunity results. Herd immunity protects those who may be immunocompromised and cannot get a vaccine because even a weakened version would harm them. The effectiveness of vaccination has been widely studied and verified. Vaccination is the most effective method of preventing infectious diseases; widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the elimination of diseases such as polio and tetanus from much of the world. According to the World Health Organization (WHO), vaccination prevents 3.5–5 million deaths per year. A WHO-funded study by The Lancet estimates that, during the 50-year period starting in 1974, vaccination prevented 154 million deaths, including 146 million among children under age 5. However, some diseases have seen rising cases due to relatively low vaccination rates attributable partly to vaccine hesitancy.

The smallpox vaccine is used to prevent smallpox infection caused by the variola virus. It is the first vaccine to have been developed against a contagious disease. In 1796, British physician Edward Jenner demonstrated that an infection with the relatively mild cowpox virus conferred immunity against the deadly smallpox virus. Cowpox served as a natural vaccine until the modern smallpox vaccine emerged in the 20th century. From 1958 to 1977, the World Health Organization (WHO) conducted a global vaccination campaign that eradicated smallpox, making it the only human disease to be eradicated. Although routine smallpox vaccination is no longer performed on the general public, the vaccine is still being produced for research, and to guard against bioterrorism, biological warfare, and mpox.

Vaccine hesitancy is a delay in acceptance, or refusal, of vaccines despite the availability of vaccine services and supporting evidence. The term covers refusals to vaccinate, delaying vaccines, accepting vaccines but remaining uncertain about their use, or using certain vaccines but not others. Although adverse effects associated with vaccines are occasionally observed, the scientific consensus that vaccines are generally safe and effective is overwhelming. Vaccine hesitancy often results in disease outbreaks and deaths from vaccine-preventable diseases. Therefore, the World Health Organization characterizes vaccine hesitancy as one of the top ten global health threats.
A breakthrough infection is a case of illness in which a vaccinated individual becomes infected with the illness, because the vaccine has failed to provide complete immunity against the pathogen. Breakthrough infections have been identified in individuals immunized against a variety of diseases including mumps, varicella (Chickenpox), influenza, and COVID-19. The characteristics of the breakthrough infection are dependent on the virus itself. Often, infection of the vaccinated individual results in milder symptoms and shorter duration than if the infection were contracted naturally.
A vaccination policy is a health policy adopted in order to prevent the spread of infectious disease. These policies are generally put into place by state or local governments, but may also be set by private facilities, such as workplaces or schools. Many policies have been developed and implemented since vaccines were first made widely available.

Pertussis vaccine is a vaccine that protects against whooping cough (pertussis). There are two main types: whole-cell vaccines and acellular vaccines. The whole-cell vaccine is about 78% effective while the acellular vaccine is 71–85% effective. The effectiveness of the vaccines appears to decrease by between 2 and 10% per year after vaccination with a more rapid decrease with the acellular vaccines. The vaccine is only available in combination with tetanus and diphtheria vaccines. Pertussis vaccine is estimated to have saved over 500,000 lives in 2002.

The Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. It completed Phase III trials in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru, and the United Arab Emirates (UAE) with over 60,000 participants. BBIBP-CorV shares similar technology with CoronaVac and Covaxin, other inactivated virus vaccines for COVID-19. Its product name is SARS-CoV-2 Vaccine, not to be confused with the similar product name of CoronaVac.
Misinformation related to immunization and the use of vaccines circulates in mass media and social media in spite of the fact that there is no serious hesitancy or debate within mainstream medical and scientific circles about the benefits of vaccination. Unsubstantiated safety concerns related to vaccines are often presented on the internet as being scientific information. A large proportion of internet sources on the topic are mostly inaccurate which can lead people searching for information to form misconceptions relating to vaccines.

Israel's COVID-19 vaccination programme, officially named "Give a Shoulder", began on 19 December 2020, and has been praised for its speed, having given twenty percent of the Israeli population the first dose of the vaccines' two dose regimen in the span of three weeks.

EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the Russian VECTOR Center of Virology. The lack of protective effectiveness of EpiVacCorona, which is still in use in Russia, has been reported in scientific literature and in the media. The vaccine consists of three chemically synthesized peptides that are conjugated to a large carrier protein. This protein is a fusion product of a viral nucleocapsid protein and a bacterial MBP protein. A phase III clinical trial to show whether or not the vaccine can protect people against COVID-19 was launched in November 2020 with more than three thousand participants. The conclusions and results of the trial have not been made public.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
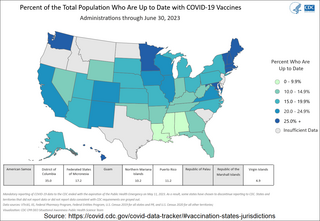
The COVID-19 vaccination campaign in the United States is an ongoing mass immunization campaign for the COVID-19 pandemic in the United States. The Food and Drug Administration (FDA) first granted emergency use authorization to the Pfizer–BioNTech vaccine on December 10, 2020, and mass vaccinations began four days later. The Moderna vaccine was granted emergency use authorization on December 17, 2020, and the Janssen vaccine was granted emergency use authorization on February 27, 2021. It was not until April 19, 2021, that all U.S. states had opened vaccine eligibility to residents aged 16 and over. On May 10, 2021, the FDA approved the Pfizer-BioNTech vaccine for adolescents aged 12 to 15. On August 23, 2021, the FDA granted full approval to the Pfizer–BioNTech vaccine for individuals aged 16 and over.

Abdala, technical name CIGB-66, is a COVID-19 vaccine developed by the Center for Genetic Engineering and Biotechnology in Cuba. This candidate, named after a patriotic drama by Cuban independence hero José Martí, is a protein subunit vaccine containing COVID-derived proteins that trigger an immune response. The full results of the clinical trial have not yet been published. This candidate followed a previous one called CIGB-669 (MAMBISA).

V-01 is a protein subunit COVID-19 vaccine candidate developed by a subsidiary of Livzon Pharmaceutical Group Inc.

A COVID-19 vaccine card is a record often given to those who have received a COVID-19 vaccine showing information such as the date(s) one has received the shot(s) and the brand of vaccine one has received, sometimes including the lot number. The card also contains information identifying the recipient and the location where the shot was given. Depending on the country, it could serve as an official document verifying one has received vaccination, which could be required by some institutions, such as a school or workplace, when boarding a cruise ship, or when crossing an international border, as proof that one has been vaccinated.

COVID-19 vaccine hesitancy in the United States is the sociocultural phenomenon of individuals refusing or displaying hesitance towards receiving the COVID-19 vaccine. COVID-19 vaccine hesitancy in the United States can be considered as part of the broader history of vaccine hesitancy.

In many countries a variety of unfounded conspiracy theories and other misinformation about COVID-19 vaccines have spread based on misunderstood or misrepresented science, religion, and law. These have included exaggerated claims about side effects, misrepresentations about how the immune system works and when and how COVID-19 vaccines are made, a story about COVID-19 being spread by 5G, and other false or distorted information. This misinformation, some created by anti-vaccination activists, has proliferated and may have made many people averse to vaccination. This has led to governments and private organizations around the world introducing measures to incentivize or coerce vaccination, such as lotteries, mandates, and free entry to events, which has in turn led to further misinformation about the legality and effect of these measures themselves.

Over the course of the COVID-19 pandemic, COVID-19 vaccine mandates have been enacted by numerous states and municipalities in the United States, and also by private entities. In September 2021, President Joe Biden announced that the federal government would take steps to mandate COVID-19 vaccination for certain entities under the authority of the federal government or federal agencies. Most federal mandates thus imposed were either overturned through litigation, or withdrawn by the administration, although a mandate on health care workers in institutions receiving Medicare and Medicaid funds was upheld. All federal mandates were lifted when the national emergency was declared to have ended in May 2023. A small number of states have gone in the opposite direction, through executive orders or legislation designed to limit vaccination mandates.
Media coverage of the COVID-19 pandemic includes reporting on the deaths of anti-vaccine advocates from COVID-19 as a phenomenon occurring during the COVID-19 pandemic. The media also reported on various websites documenting such deaths, with some outlets questioning whether this practice was overly unsympathetic. Reports noted phenomena including "deathbed conversions", in which vaccine opponents reportedly changed their minds and began encouraging vaccination before dying, with these claims meeting continued skepticism by vaccination opponents; and on groups of deaths within specific demographics, such as anti-vaccine radio hosts.

The Exposé is a British conspiracist and fake news website created in 2020 by Jonathan Allen-Walker. It is known for publishing COVID-19 and anti-vaccine misinformation.












