Related Research Articles
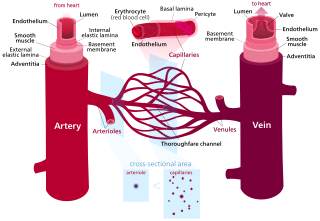
Blood vessels are the tubular structures of a circulatory system that transport blood throughout a vertebrate's body. Blood vessels transport blood cells, nutrients, and oxygen to most of the tissues of a body. They also take waste and carbon dioxide away from the tissues. Some tissues such as cartilage, epithelium, and the lens and cornea of the eye are not supplied with blood vessels and are termed avascular.

Veins are blood vessels in the circulatory system of humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are those of the pulmonary and fetal circulations which carry oxygenated blood to the heart. In the systemic circulation, arteries carry oxygenated blood away from the heart, and veins return deoxygenated blood to the heart, in the deep veins.

A capillary is a small blood vessel, from 5 to 10 micrometres in diameter, and is part of the microcirculation system. Capillaries are microvessels and the smallest blood vessels in the body. They are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the site of the exchange of many substances from the surrounding interstitial fluid, and they convey blood from the smallest branches of the arteries (arterioles) to those of the veins (venules). Other substances which cross capillaries include water, oxygen, carbon dioxide, urea, glucose, uric acid, lactic acid and creatinine. Lymph capillaries connect with larger lymph vessels to drain lymphatic fluid collected in microcirculation.

The mitral valve, also known as the bicuspid valve or left atrioventricular valve, is one of the four heart valves. It has two cusps or flaps and lies between the left atrium and the left ventricle of the heart. The heart valves are all one-way valves allowing blood flow in just one direction. The mitral valve and the tricuspid valve are known as the atrioventricular valves because they lie between the atria and the ventricles.

Cardiac muscle is one of three types of vertebrate muscle tissues, the others being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall and the inner layer, with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.

Mitral valve prolapse (MVP) is a valvular heart disease characterized by the displacement of an abnormally thickened mitral valve leaflet into the left atrium during systole. It is the primary form of myxomatous degeneration of the valve. There are various types of MVP, broadly classified as classic and nonclassic. In severe cases of classic MVP, complications include mitral regurgitation, infective endocarditis, congestive heart failure, and, in rare circumstances, cardiac arrest.

The endocardium is the innermost layer of tissue that lines the chambers of the heart. Its cells are embryologically and biologically similar to the endothelial cells that line blood vessels. The endocardium also provides protection to the valves and heart chambers.
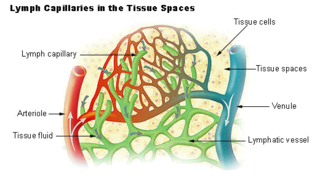
The lymphatic vessels are thin-walled vessels (tubes), structured like blood vessels, that carry lymph. As part of the lymphatic system, lymph vessels are complementary to the cardiovascular system. Lymph vessels are lined by endothelial cells, and have a thin layer of smooth muscle, and adventitia that binds the lymph vessels to the surrounding tissue. Lymph vessels are devoted to the propulsion of the lymph from the lymph capillaries, which are mainly concerned with the absorption of interstitial fluid from the tissues. Lymph capillaries are slightly bigger than their counterpart capillaries of the vascular system. Lymph vessels that carry lymph to a lymph node are called afferent lymph vessels, and those that carry it from a lymph node are called efferent lymph vessels, from where the lymph may travel to another lymph node, may be returned to a vein, or may travel to a larger lymph duct. Lymph ducts drain the lymph into one of the subclavian veins and thus return it to general circulation.

The glycocalyx, also known as the pericellular matrix and cell coat, is a layer of glycoproteins and glycolipids which surround the cell membranes of bacteria, epithelial cells, and other cells.

The interstitium is a contiguous fluid-filled space existing between a structural barrier, such as a cell membrane or the skin, and internal structures, such as organs, including muscles and the circulatory system. The fluid in this space is called interstitial fluid, comprises water and solutes, and drains into the lymph system. The interstitial compartment is composed of connective and supporting tissues within the body – called the extracellular matrix – that are situated outside the blood and lymphatic vessels and the parenchyma of organs. The role of the interstitium in solute concentration, protein transport and hydrostatic pressure impacts human pathology and physiological responses such as edema, inflammation and shock.
Cardiomyoplasty is a surgical procedure in which healthy muscle from another part of the body is wrapped around the heart to provide support for the failing heart. Most often the latissimus dorsi muscle is used for this purpose. A special pacemaker is implanted to make the skeletal muscle contract. If cardiomyoplasty is successful and increased cardiac output is achieved, it usually acts as a bridging therapy, giving time for damaged myocardium to be treated in other ways, such as remodeling by cellular therapies.
Cardiac fibrosis commonly refers to the excess deposition of extracellular matrix in the cardiac muscle, but the term may also refer to an abnormal thickening of the heart valves due to inappropriate proliferation of cardiac fibroblasts. Fibrotic cardiac muscle is stiffer and less compliant and is seen in the progression to heart failure. The description below focuses on a specific mechanism of valvular pathology but there are other causes of valve pathology and fibrosis of the cardiac muscle.

Periostin is a protein that in humans is encoded by the POSTN gene. Periostin functions as a ligand for alpha-V/beta-3 and alpha-V/beta-5 integrins to support adhesion and migration of epithelial cells.
Amniotic stem cells are the mixture of stem cells that can be obtained from the amniotic fluid as well as the amniotic membrane. They can develop into various tissue types including skin, cartilage, cardiac tissue, nerves, muscle, and bone. The cells also have potential medical applications, especially in organ regeneration.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
Decellularization of porcine heart valves is the removal of cells along with antigenic cellular elements by either physical or chemical decellularization of the tissue. This decellularized valve tissue provides a scaffold with the remaining extracellular matrix (ECM) that can then be used for tissue engineering and valve replacement in humans inflicted with valvular disease. Decellularized biological valves have potential benefit over conventional valves through decreased calcification which is thought to be an immuno-inflammatory response initiated by the recipient.
Neural crest cells are multipotent cells required for the development of cells, tissues and organ systems. A subpopulation of neural crest cells are the cardiac neural crest complex. This complex refers to the cells found amongst the midotic placode and somite 3 destined to undergo epithelial-mesenchymal transformation and migration to the heart via pharyngeal arches 3, 4 and 6.
Robert M. Nerem, often referred to as Bob Nerem, a member of the U. S. National Academy of Engineering and the Institute of Medicine, held the Parker H. Petit Distinguished Chair for Engineering in Medicine and Institute Professor Emeritus at the Georgia Institute of Technology where he was an Emeritus Professor until his death.
Tissue engineered heart valves (TEHV) offer a new and advancing proposed treatment of creating a living heart valve for people who are in need of either a full or partial heart valve replacement. Currently, there are over a quarter of a million prosthetic heart valves implanted annually, and the number of patients requiring replacement surgeries is only suspected to rise and even triple over the next fifty years. While current treatments offered such as mechanical valves or biological valves are not deleterious to one's health, they both have their own limitations in that mechanical valves necessitate the lifelong use of anticoagulants while biological valves are susceptible to structural degradation and reoperation. Thus, in situ (in its original position or place) tissue engineering of heart valves serves as a novel approach that explores the use creating a living heart valve composed of the host's own cells that is capable of growing, adapting, and interacting within the human body's biological system.
References
- ↑ Taylor, Patricia M; Batten, Puspa; Brand, Nigel J; Thomas, Penny S; Yacoub, Magdi H (2003-02-01). "The cardiac valve interstitial cell". The International Journal of Biochemistry & Cell Biology. 35 (2): 113–118. doi:10.1016/S1357-2725(02)00100-0. ISSN 1357-2725. PMID 12479860.
- 1 2 3 Rutkovskiy, Arkady; Malashicheva, Anna; Sullivan, Gareth; Bogdanova, Maria; Kostareva, Anna; Stensløkken, Kåre-Olav; Fiane, Arnt; Vaage, Jarle (2017-09-22). "Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification". Journal of the American Heart Association. 6 (9). doi:10.1161/JAHA.117.006339. ISSN 2047-9980. PMC 5634284 . PMID 28912209.
- ↑ Hinton, Robert B.; Yutzey, Katherine E. (2011). "Heart Valve Structure and Function in Development and Disease". Annual Review of Physiology. 73: 29–46. doi:10.1146/annurev-physiol-012110-142145. ISSN 0066-4278. PMC 4209403 . PMID 20809794.
- ↑ Ground, Marcus; Callon, Karen; Walker, Rob; Milsom, Paget; Cornish, Jillian (2023-09-18), "Valvular Interstitial Cells: Physiology, Isolation, and Culture", Biochemistry, vol. 0, IntechOpen, doi: 10.5772/intechopen.112649 , retrieved 2024-02-12