Related Research Articles

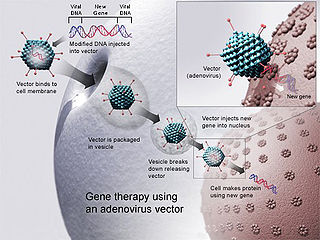
Gene therapy is a medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.
Dyslipidemia is a metabolic disorder characterized by abnormally high or low amounts of any or all lipids or lipoproteins in the blood. Dyslipidemia is a risk factor for the development of atherosclerotic cardiovascular diseases, which include coronary artery disease, cerebrovascular disease, and peripheral artery disease. Although dyslipidemia is a risk factor for cardiovascular disease, abnormal levels do not mean that lipid lowering agents need to be started. Other factors, such as comorbid conditions and lifestyle in addition to dyslipidemia, is considered in a cardiovascular risk assessment. In developed countries, most dyslipidemias are hyperlipidemias; that is, an elevation of lipids in the blood. This is often due to diet and lifestyle. Prolonged elevation of insulin resistance can also lead to dyslipidemia.
Human genetic enhancement or human genetic engineering refers to human enhancement by means of a genetic modification. This could be done in order to cure diseases, prevent the possibility of getting a particular disease, to improve athlete performance in sporting events, or to change physical appearance, metabolism, and even improve physical capabilities and mental faculties such as memory and intelligence. These genetic enhancements may or may not be done in such a way that the change is heritable.

A designer baby is a baby whose genetic makeup has been selected or altered, often to exclude a particular gene or to remove genes associated with disease. This process usually involves analysing a wide range of human embryos to identify genes associated with particular diseases and characteristics, and selecting embryos that have the desired genetic makeup; a process known as preimplantation genetic diagnosis. Screening for single genes is commonly practiced, and polygenic screening is offered by a few companies. Other methods by which a baby's genetic information can be altered involve directly editing the genome before birth, which is not routinely performed and only one instance of this is known to have occurred as of 2019, where Chinese twins Lulu and Nana were edited as embryos, causing widespread criticism.
Virotherapy is a treatment using biotechnology to convert viruses into therapeutic agents by reprogramming viruses to treat diseases. There are three main branches of virotherapy: anti-cancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy. These branches use three different types of treatment methods: gene overexpression, gene knockout, and suicide gene delivery. Gene overexpression adds genetic sequences that compensate for low to zero levels of needed gene expression. Gene knockout uses RNA methods to silence or reduce expression of disease-causing genes. Suicide gene delivery introduces genetic sequences that induce an apoptotic response in cells, usually to kill cancerous growths. In a slightly different context, virotherapy can also refer more broadly to the use of viruses to treat certain medical conditions by killing pathogens.
A CETP inhibitor is a member of a class of drugs that inhibit cholesterylester transfer protein (CETP). They are intended to reduce the risk of atherosclerosis by improving blood lipid levels. At least three medications within this class have failed to demonstrate a beneficial effect.

Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein cholesterol, in the blood and early cardiovascular diseases. The most common mutations diminish the number of functional LDL receptors in the liver or produce abnormal LDL receptors that never go to the cell surface to function properly. Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH. Nevertheless, treatment is usually effective.

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme encoded by the PCSK9 gene in humans on chromosome 1. It is the 9th member of the proprotein convertase family of proteins that activate other proteins. Similar genes (orthologs) are found across many species. As with many proteins, PCSK9 is inactive when first synthesized, because a section of peptide chains blocks their activity; proprotein convertases remove that section to activate the enzyme. The PCSK9 gene also contains one of 27 loci associated with increased risk of coronary artery disease.

Santaris Pharma A/S was a biopharmaceutical company founded in 2003 in Copenhagen, Denmark. The company also had a branch in San Diego, California that opened in 2009. Created by a merger between Cureon and Pantheco, Santaris developed RNA-targeted medicines using a Locked Nucleic Acid (LNA) Drug Platform and Drug Development Engine.

Genome editing, or genome engineering, or gene editing, is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living organism. Unlike early genetic engineering techniques that randomly inserts genetic material into a host genome, genome editing targets the insertions to site-specific locations. The basic mechanism involved in genetic manipulations through programmable nucleases is the recognition of target genomic loci and binding of effector DNA-binding domain (DBD), double-strand breaks (DSBs) in target DNA by the restriction endonucleases, and the repair of DSBs through homology-directed recombination (HDR) or non-homologous end joining (NHEJ).
Alirocumab, sold under the brand name Praluent, is a medication used as a second-line treatment for high cholesterol for adults whose cholesterol is not controlled by diet and statin treatment. It is a human monoclonal antibody that belongs to a novel class of anti-cholesterol drugs, known as PCSK9 inhibitors, and it was the first such agent to receive FDA approval. The FDA approval was contingent on the completion of further clinical trials to better determine efficacy and safety.
Evolocumab, sold under the brand name Repatha, is a monoclonal antibody that is an immunotherapy medication for the treatment of hyperlipidemia.

Editas Medicine, Inc.,, is a clinical-stage biotechnology company which is developing therapies for rare diseases based on CRISPR gene editing technology. Editas headquarters is located in Cambridge, Massachusetts and has facilities in Boulder, Colorado.
Inclisiran, sold under the brand name Leqvio, is a medication used for the treatment of high low-density lipoprotein (LDL) cholesterol and for the treatment of people with atherosclerotic cardiovascular disease (ASCVD), ASCVD risk-equivalents, and heterozygous familial hypercholesterolemia (HeFH). It is a small interfering RNA (siRNA) that acts as an inhibitor of a proprotein convertase, specifically, inhibiting translation of the protein PCSK9.

Intellia Therapeutics, Inc. is an American clinical-stage biotechnology company focused on developing novel, potentially curative therapeutics leveraging CRISPR-based technologies. The company's in vivo programs use intravenously administered CRISPR as the therapy, in which the company's proprietary delivery technology enables highly precise editing of disease-causing genes directly within specific target tissues. Intellia's ex vivo programs use CRISPR to create the therapy by using engineered human cells to treat cancer and autoimmune diseases.

CRISPR gene editing is a genetic engineering technique in molecular biology by which the genomes of living organisms may be modified. It is based on a simplified version of the bacterial CRISPR-Cas9 antiviral defense system. By delivering the Cas9 nuclease complexed with a synthetic guide RNA (gRNA) into a cell, the cell's genome can be cut at a desired location, allowing existing genes to be removed and/or new ones added in vivo.

CRISPR Therapeutics AG is a Swiss–American biotechnology company headquartered in Zug, Switzerland. It was one of the first companies formed to utilize the CRISPR gene editing platform to develop medicines for the treatment of various rare and common diseases. The company has approximately 500 employees and has offices in Zug, Switzerland, Boston, Massachusetts, San Francisco, California and London, United Kingdom. Its manufacturing facility in Framingham, Massachusetts won the Facilities of the Year Award (FOYA) award in 2022. The company’s lead program, exagamglogene autotemcel, or exa-cel, was granted regulatory approval by the US Food and Drug Administration (FDA) in December 2023.
Kiran Musunuru is an American cardiologist who is a Professor of Medicine at the University of Pennsylvania Perelman School of Medicine. He researches the genetics and genomics of cardiovascular and metabolic diseases. Musunuru is a leading expert in the field of gene-editing.
Precision BioSciences, Inc. is a publicly traded American clinical stage gene editing company headquartered in Durham, North Carolina. Founded in 2006, Precision is focused on developing both in vivo and ex vivo gene editing therapies using its proprietary "ARCUS" genome editing platform.
The Innovative Genomics Institute (IGI) is an American nonprofit scientific research institute founded by Nobel laureate and CRISPR gene editing pioneer Jennifer Doudna and biophysicist Jonathan Weissman. The institute is based at the University of California, Berkeley, and also has member researchers at the University of California, San Francisco, UC Davis, UCLA, Lawrence Berkeley National Laboratory, Lawrence Livermore National Laboratory, Gladstone Institutes, and other collaborating research institutions. The IGI focuses on developing real-world applications of genome editing to address problems in human health, agriculture and climate change.
References
- ↑ "Next up for CRISPR: Gene editing for the masses?". MIT Technology Review. Retrieved 5 December 2023.
- ↑ Philippidis, Alex (1 January 2023). "StockWatch: Verve Investors Accentuate the Negative as Shares Tumble, Then Recover: Safety concerns propel 40% drop before stock bounces back as analysts, researchers focus on VERVE-101's positive human proof-of-concept data". GEN Edge. 5 (1): 808–813. doi:10.1089/genedge.5.1.153. S2CID 265960415.
- ↑ Lewis, Basil S (29 November 2023). "First-in-human trial of PCSK9 gene editing therapy for lowering cholesterol: a new frontier in cardiovascular pharmacotherapy? News from AHA". European Heart Journal - Cardiovascular Pharmacotherapy. 10 (2): 87–88. doi: 10.1093/ehjcvp/pvad095 . PMID 38031331.
- ↑ Knutsen, Ashleen (1 March 2022). "Opening New Lines of Attack against Cardiovascular Disease: Developers of cardiovascular drugs hope to disrupt newly identified molecular mechanisms by deploying highly specific small-molecule drugs and gene therapies". Genetic Engineering & Biotechnology News. 42 (3): 62–65. doi:10.1089/gen.42.03.19. S2CID 247339058.
- ↑ Lee, Richard G.; Mazzola, Anne Marie; Braun, Maurine C.; Platt, Colin; Vafai, Scott B.; Kathiresan, Sekar; Rohde, Ellen; Bellinger, Andrew M.; Khera, Amit V. (17 January 2023). "Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models". Circulation. 147 (3): 242–253. doi: 10.1161/CIRCULATIONAHA.122.062132 . PMID 36314243.
- ↑ "Verve pauses gene therapy trial, switches delivery agent". Chemical & Engineering News.