Related Research Articles

The Food and Drug Administration is a federal agency of the United States Department of Health and Human Services, one of the United States federal executive departments. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.

A prescription drug is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug.

There are different systems of feeding cattle in animal husbandry, which may have different advantages and disadvantages. Most cattle in the US have a fodder that is composed of at least some forage. In fact, most beef cattle are raised on pasture from birth in the spring until autumn. For pastured animals, grass is usually the forage that composes the majority of their diet. Cattle reared in feedlots are fed hay supplemented with grain, soy and other ingredients in order to increase the energy density of the feed. The debate is whether cattle should be raised on fodder primarily composed of grass or a concentrate. The issue is complicated by the political interests and confusion between labels such as "free range", "organic", or "natural". Cattle raised on a primarily foraged diet are termed grass-fed or pasture-raised; for example meat or milk may be called grass-fed beef or pasture-raised dairy. The term "pasture-raised" can lead to confusion with the term "free range", which does not describe exactly what the animals eat.

The regulation of therapeutic goods, defined as drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United States, they are regulated at the national level by a single agency. In other jurisdictions they are regulated at the state level, or at both state and national levels by various bodies, as in Australia.

Off-label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration. Both prescription drugs and over-the-counter drugs (OTCs) can be used in off-label ways, although most studies of off-label use focus on prescription drugs.
Raw feeding is the practice of feeding domestic dogs, cats and other animals a diet consisting primarily of uncooked meat, edible bones, and organs. The ingredients used to formulate raw diets can vary. Some pet owners choose to make home-made raw diets to feed their animals but commercial raw food diets are also available.
Beginning in March 2007, there was a wide recall of many brands of cat and dog foods due to contamination with melamine and cyanuric acid. The recalls in North America, Europe, and South Africa came in response to reports of renal failure in pets. Initially, the recalls were associated with the consumption of mostly wet pet foods made with wheat gluten from a single Chinese company. After more than three weeks of complaints from consumers, the recall began voluntarily with the Canadian company Menu Foods on 16 March 2007, when a company test showed sickness and death in some of the test animals. Soon after, there were numerous media reports of animal deaths as a result of kidney failure. In the following weeks, several other companies who received the contaminated wheat gluten also voluntarily recalled dozens of pet food brands. One month after the initial recall, contaminated rice protein from a different source in China was also identified as being associated with kidney failure in pets in the United States, while contaminated corn gluten was associated with kidney failure with pets in South Africa. As a result of investigating the 2007 pet food recalls, a broader Chinese protein export contamination investigation unfolded, raising concerns about the safety of the human food supply.
This timeline of the 2007 pet food recalls documents how events related to the 2007 pet food recalls unfolded. Several contaminated Chinese vegetable proteins were used by pet food makers in North America, Europe and South Africa, leading to renal failure in animals fed the contaminated food. Both the centralization of the pet food industry and the speed and manner of the industry and government response became the subjects of critical discussion.
In China, the adulteration and contamination of several food and feed ingredients with inexpensive melamine and other compounds, such as cyanuric acid, ammeline and ammelide, are common practice. These adulterants can be used to inflate the apparent protein content of products, so that inexpensive ingredients can pass for more expensive, concentrated proteins. Melamine by itself has not been thought to be very toxic to animals or humans except possibly in very high concentrations, but the combination of melamine and cyanuric acid has been implicated in kidney failure. Reports that cyanuric acid may be an independently and potentially widely used adulterant in China have heightened concerns for both animal and human health.
Organic beef is beef grown according to organic food principles.
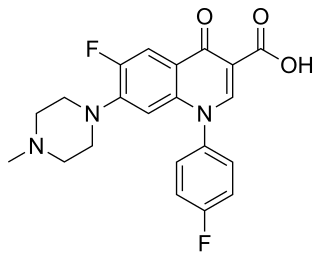
Difloxacin (INN), marketed under the trade name Dicural, is a second-generation, synthetic fluoroquinolone antibiotic used in veterinary medicine. It has broad-spectrum, concentration dependent, bactericidal activity; however, its efficacy is not as good as enrofloxacin or pradofloxacin.
The United States is the largest grower of commercial crops that have been genetically engineered in the world, but not without domestic and international opposition.

Antibiotic misuse, sometimes called antibiotic abuse or antibiotic overuse, refers to the misuse or overuse of antibiotics, with potentially serious effects on health. It is a contributing factor to the development of antibiotic resistance, including the creation of multidrug-resistant bacteria, informally called "super bugs": relatively harmless bacteria can develop resistance to multiple antibiotics and cause life-threatening infections.
A feed additive is an additive of extra nutrient or drug for livestock. Such additives include vitamins, amino acids, fatty acids, minerals, pharmaceutical, fungal products and steroidal compounds. The additives might impact feed presentation, hygiene, digestibility, or effect on intestinal health.

The Animal Drug Availability Act 1996 (ADAA) is a United States federal law. President Clinton signed the ADAA into law in October 1996. While still obligated to public health concerns, the Act intends more rapid drug approval and medicated feed approval to assist the animal health industry.
Antimicrobials destroy bacteria, viruses, fungi, algae, and other microbes. The cells of bacteria (prokaryotes), such as salmonella, differ from those of higher-level organisms (eukaryotes), such as fish. Antibiotics are chemicals designed to either kill or inhibit the growth of pathogenic bacteria while exploiting the differences between prokaryotes and eukaryotes in order to make them relatively harmless in higher-level organisms. Antibiotics are constructed to act in one of three ways: by disrupting cell membranes of bacteria, by impeding DNA or protein synthesis, or by hampering the activity of certain enzymes unique to bacteria.
Antibiotic use in livestock is the use of antibiotics for any purpose in the husbandry of livestock, which includes treatment when ill (therapeutic), treatment of a herd of animals when at least one is diagnosed as ill (metaphylaxis), and preventative treatment (prophylaxis). The use of subtherapeutic doses in animal feed and water to promote growth and improve feed efficiency became illegal in the United States on 1 January 2017, through legislative change enacted by the Food and Drug Administration (FDA), which sought voluntary compliance from drug manufacturers to re-label their antibiotics. The use of antibiotics in livestock including poultry may have impacts on human and environmental health. Legislation on antibiotic use in farm animals is being introduced across the globe.
In the United StatesVeterinary pharmacy is a field of pharmacy practice, in which veterinary pharmacists may compound medications, fill prescriptions, and manage drug therapies for animals. Veterinary pharmacists are licensed pharmacists who specialize in the distribution of medications for animals. This differs slightly from the title of "veterinary pharmacy specialist," who might additionally work in consulting, research, and education for veterinary pharmacy. Regular pharmacists in a variety of settings come into play in the preparation and dispensing of animal medications as well. As veterinarians treat a wide variety of animals with a wide variety of products, pharmacists can help manage these treatments through their compounding and drug knowledge. Compounding is often necessary for animal patients, as they require different dosages and medication forms from humans. Through compounding, pharmacists can adjust a medication for an animal so it is more appealing in taste or appearance. While there is currently no required veterinary pharmacy curriculum in place by the Accreditation Council for Pharmacy Education, the American Veterinary Medical Association understands that some veterinary education might be beneficial to pharmacists as community pharmacies continue to supply animal medications.
Medicated feed is an American legal terminology. As of 2019, it was defined in 21 CFR § 558.3.
Antibiotics in poultry farming is the controversial prophylactic use of antibiotics in the poultry farming industry. They have been applied in mass quantities since 1951, when the U.S. Food and Drug Administration (FDA) approved their use. Three years prior to the FDA's approval, scientists were investigating a phenomenon in which chickens who were rooting through bacteria-rich manure were displaying signs of greater health than those who did not. Through testing, it was discovered that chickens who were fed a variety of vitamin B12 manufactured with the residue of a certain antibiotic grew 50 percent faster than those chickens who were fed B12 manufactured from a different source. Further testing confirmed that use of antibiotics did improve the health of the chickens, resulting in the chickens laying more eggs and experiencing lower mortality rates and less illness. Upon this discovery, farmers transitioned from expensive animal proteins to comparatively inexpensive antibiotics and B12. Chickens were now reaching their market weight at a much faster rate and at a lower cost. With a growing population and greater demand on the farmers, antibiotics appeared to be an ideal and cost-effective way to increase the output of poultry. Since this discovery, antibiotics have been routinely used in poultry production, but more recently have been the topic of debate secondary to the fear of bacterial antibiotic resistance.
References
- 1 2 3 4 Womach, Jasper (2005). Report for Congress: Agriculture: A Glossary of Terms, Programs, and Laws (PDF) (Report). Congressional Research Service. p. 271. Archived from the original (PDF) on 2011-08-10.
- ↑ "Veterinary Feed Directive (VFD) Basics". American Veterinary Medical Association. Retrieved 28 June 2019.
- ↑ "Veterinarian-Client-Patient Relationship (VCPR)". American Veterinary Medical Association. Retrieved 2019-06-28.
- ↑ [aast.cfsph.iastate.edu/VFD/index.htm.VFD/index.htm]
- ↑ "National Veterinary Accreditation Program". USDA. Retrieved 28 June 2019.
- ↑ "FDA Reminds Retail Establishments of Upcoming Changes to the Use of Antibiotics in Food Animals". U.S. Food and Drug Administration. Archived from the original on 2017-01-11.