
Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
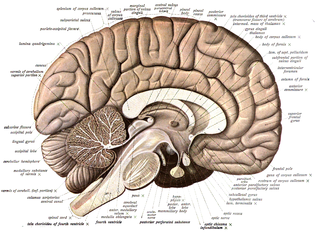
Neuroanatomy is the study of the structure and organization of the nervous system. In contrast to animals with radial symmetry, whose nervous system consists of a distributed network of cells, animals with bilateral symmetry have segregated, defined nervous systems. Their neuroanatomy is therefore better understood. In vertebrates, the nervous system is segregated into the internal structure of the brain and spinal cord and the series of nerves that connect the CNS to the rest of the body. Breaking down and identifying specific parts of the nervous system has been crucial for figuring out how it operates. For example, much of what neuroscientists have learned comes from observing how damage or "lesions" to specific brain areas affects behavior or other neural functions.
A histochemical tracer is a compound used to reveal the location of cells and track neuronal projections. A neuronal tracer may be retrograde, anterograde, or work in both directions. A retrograde tracer is taken up in the terminal of the neuron and transported to the cell body, whereas an anterograde tracer moves away from the cell body of the neuron.
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in animals, including humans. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries and on all continents except Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

A neural circuit is a population of neurons interconnected by synapses to carry out a specific function when activated. Multiple neural circuits interconnect with one another to form large scale brain networks.

Axonal transport, also called axoplasmic transport or axoplasmic flow, is a cellular process responsible for movement of mitochondria, lipids, synaptic vesicles, proteins, and other organelles to and from a neuron's cell body, through the cytoplasm of its axon called the axoplasm. Since some axons are on the order of meters long, neurons cannot rely on diffusion to carry products of the nucleus and organelles to the end of their axons. Axonal transport is also responsible for moving molecules destined for degradation from the axon back to the cell body, where they are broken down by lysosomes.
Aujeszky's disease, usually called pseudorabies in the United States, is a viral disease in swine that is endemic in most parts of the world. It is caused by Suid herpesvirus 1 (SuHV-1). Aujeszky's disease is considered to be the most economically important viral disease of swine in areas where classical swine fever has been eradicated. Other mammals, such as cattle, sheep, goats, cats, dogs, and raccoons, are also susceptible. The disease is usually fatal in these animal species.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

Satellite glial cells, formerly called amphicytes, are glial cells that cover the surface of neuron cell bodies in ganglia of the peripheral nervous system. Thus, they are found in sensory, sympathetic, and parasympathetic ganglia. Both satellite glial cells (SGCs) and Schwann cells are derived from the neural crest of the embryo during development. SGCs have been found to play a variety of roles, including control over the microenvironment of sympathetic ganglia. They are thought to have a similar role to astrocytes in the central nervous system (CNS). They supply nutrients to the surrounding neurons and also have some structural function. Satellite cells also act as protective, cushioning cells. Additionally, they express a variety of receptors that allow for a range of interactions with neuroactive chemicals. Many of these receptors and other ion channels have recently been implicated in health issues including chronic pain and herpes simplex. There is much more to be learned about these cells, and research surrounding additional properties and roles of the SGCs is ongoing.

Synaptic pruning, a phase in the development of the nervous system, is the process of synapse elimination that occurs between early childhood and the onset of puberty in many mammals, including humans. Pruning starts near the time of birth and continues into the late-20s. During pruning, both the axon and dendrite decay and die off. It was traditionally considered to be complete by the time of sexual maturation, but this was discounted by MRI studies.

The Calyx of Held is a particularly large synapse in the mammalian auditory central nervous system, so named after Hans Held who first described it in his 1893 article Die centrale Gehörleitung because of its resemblance to the calyx of a flower. Globular bushy cells in the anteroventral cochlear nucleus (AVCN) send axons to the contralateral medial nucleus of the trapezoid body (MNTB), where they synapse via these calyces on MNTB principal cells. These principal cells then project to the ipsilateral lateral superior olive (LSO), where they inhibit postsynaptic neurons and provide a basis for interaural level detection (ILD), required for high frequency sound localization. This synapse has been described as the largest in the brain.
In neuroscience, anterograde tracing is a research method that is used to trace axonal projections from their source to their point of termination. A hallmark of anterograde tracing is the labeling of the presynaptic and the postsynaptic neuron(s). The crossing of the synaptic cleft is a vital difference between the anterograde tracers and the dye fillers used for morphological reconstruction. The complementary technique is retrograde tracing, which is used to trace neural connections from their termination to their source. Both the anterograde and retrograde tracing techniques are based on the visualization of the biological process of axonal transport.
Developmental plasticity is a general term referring to changes in neural connections during development as a result of environmental interactions as well as neural changes induced by learning. Much like neuroplasticity, or brain plasticity, developmental plasticity is specific to the change in neurons and synaptic connections as a consequence of developmental processes. A child creates most of these connections from birth to early childhood. There are three primary methods by which this may occur as the brain develops, but critical periods determine when lasting changes may form. Developmental plasticity may also be used in place of the term phenotypic plasticity when an organism in an embryonic or larval stage can alter its phenotype based on environmental factors. However, a main difference between the two is that phenotypic plasticity experienced during adulthood can be reversible, whereas traits that are considered developmentally plastic set foundations during early development that remain throughout the life of the organism.
Neurovirology is an interdisciplinary field which represents a melding of clinical neuroscience, virology, immunology, and molecular biology. The main focus of the field is to study viruses capable of infecting the nervous system. In addition to this, the field studies the use of viruses to trace neuroanatomical pathways, for gene therapy, and to eliminate detrimental populations of neural cells.

Retrograde tracing is a research method used in neuroscience to trace neural connections from their point of termination to their source. Retrograde tracing techniques allow for detailed assessment of neuronal connections between a target population of neurons and their inputs throughout the nervous system. These techniques allow the "mapping" of connections between neurons in a particular structure and the target neurons in the brain. The opposite technique is anterograde tracing, which is used to trace neural connections from their source to their point of termination. Both the anterograde and retrograde tracing techniques are based on the visualization of axonal transport.
Biotinylated dextran amines (BDA) are organic compounds used as anterograde and retrograde neuroanatomical tracers. They can be used for labeling the source as well as the point of termination of neural connections and therefore to study neural pathways.
Neuronal tracing, or neuron reconstruction is a technique used in neuroscience to determine the pathway of the neurites or neuronal processes, the axons and dendrites, of a neuron. From a sample preparation point of view, it may refer to some of the following as well as other genetic neuron labeling techniques,

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.









