
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are involved, with the same joints typically involved on both sides of the body. The disease may also affect other parts of the body, including skin, eyes, lungs, heart, nerves and blood. This may result in a low red blood cell count, inflammation around the lungs, and inflammation around the heart. Fever and low energy may also be present. Often, symptoms come on gradually over weeks to months.
Rheumatology is a branch of medicine devoted to the diagnosis and management of disorders whose common feature is inflammation in the bones, muscles, joints, and internal organs. Rheumatology covers more than 100 different complex diseases, collectively known as rheumatic diseases, which includes many forms of arthritis as well as lupus and Sjögren's syndrome. Doctors who have undergone formal training in rheumatology are called rheumatologists.

Methotrexate (MTX), formerly known as amethopterin, is a chemotherapy agent and immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancies. Types of cancers it is used for include breast cancer, leukemia, lung cancer, lymphoma, gestational trophoblastic disease, and osteosarcoma. Types of autoimmune diseases it is used for include psoriasis, rheumatoid arthritis, and Crohn's disease. It can be given by mouth or by injection.

Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.
Rheumatoid factor (RF) is the autoantibody that was first found in rheumatoid arthritis. It is defined as an antibody against the Fc portion of IgG and different RFs can recognize different parts of the IgG-Fc. RF and IgG join to form immune complexes that contribute to the disease process such as chronic inflammation and join destruction at the synovium and cartilage.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow injection into a vein. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
Adalimumab, sold under the brand name Humira, among others, is a monoclonal antibody used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis. It is administered by subcutaneous injection.
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans. Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system. The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients. The International Nonproprietary Names of humanized antibodies end in -zumab, as in omalizumab.
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.

Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.

Biological therapy, the use of medications called biopharmaceuticals or biologics that are tailored to specifically target an immune or genetic mediator of disease, plays a major role in the treatment of inflammatory bowel disease. Even for diseases of unknown cause, molecules that are involved in the disease process have been identified, and can be targeted for biological therapy. Many of these molecules, which are mainly cytokines, are directly involved in the immune system. Biological therapy has found a niche in the management of cancer, autoimmune diseases, and diseases of unknown cause that result in symptoms due to immune related mechanisms.
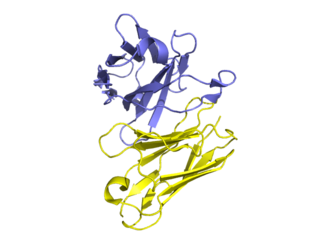
Golimumab is a human monoclonal antibody which is used as an immunosuppressive medication and sold under the brand name Simponi. Golimumab targets tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory molecule and hence is a TNF inhibitor. Profound reduction in C-reactive protein (CRP) levels, interleukin (IL)-6, intercellaular adhesion molecules (ICAM)-1, matrix metalloproteinase (MMP)-3, and vascular endothelial growth factor (VEGF) demonstrates golimumab as an effective modulator of inflammatory markers and bone metabolism.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
Etaracizumab, also known as MEDI-522, trade name Abegrin, is a humanized monoclonal antibody which is being investigated for the treatment of metastatic melanoma, prostate cancer, ovarian cancer and various other types of cancer. It is manufactured by MedImmune.

Interferon alpha-1 is a protein that in humans is encoded by the IFNA1 gene.

Anti-citrullinated protein antibodies (ACPAs) are autoantibodies that are directed against peptides and proteins that are citrullinated. They are present in the majority of patients with rheumatoid arthritis. Clinically, cyclic citrullinated peptides (CCP) are frequently used to detect these antibodies in patient serum or plasma.
Sir Marc Feldmann,, is an Australian-educated British immunologist. He is a professor at the University of Oxford and a senior research fellow at Somerville College, Oxford.
Anti-interleukin-6 agents are a class of therapeutics. Interleukin 6 is a cytokine relevant to many inflammatory diseases and many cancers. Hence, anti-IL6 agents have been sought. In rheumatoid arthritis they can help patients unresponsive to TNF inhibitors.
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony-stimulating factor receptor (GM-CSF-R).
Otilimab is a fully human antibody which has been developed by the biotechnology company MorphoSys. It can also be referred to as HuCAL antibody, HuCAL standing for Human Combinatorial Antibody Library and being a technology used to generate monoclonal antibodies. Otilimab is directed against the granulocyte-macrophage colony stimulating factor (GM-CSF), a monomeric glycoprotein functioning as a cytokine promoting both proliferation and activation of macrophages and neutrophils.







