Related Research Articles

The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. It is headquartered in Geneva, Switzerland, and has six regional offices and 150 field offices worldwide.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an initiative that brings together regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceutical product development and registration. The mission of the ICH is to promote public health by achieving greater harmonisation through the development of technical Guidelines and requirements for pharmaceutical product registration.
OFFLU is the joint OIE-FAO global network of expertise on animal influenzas. OFFLU aims to reduce the negative impacts of animal influenza viruses by promoting effective collaboration between animal health experts and the human health sector. OFFLU analyzes and shares information and biological material to identify and reduce health threats early, and shares information about animal influenza viruses with the World Health Organization (WHO) to assist with the early preparation of human vaccines. It was established in 2005, initially to support the global effort to control H5N1 highly pathogenic avian influenza.
The International Health Regulations (IHR), first adopted by the World Health Assembly in 1969 and last revised in 2005, are a legally binding rules that only apply to the WHO that is an instrument that aims for international collaboration "to prevent, protect against, control, and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risks and that avoid unnecessary interference with international traffic and trade". The IHR is the only international legal treaty with the responsibility of empowering the World Health Organization (WHO) to act as the main global surveillance system.

Project 112 was a biological and chemical weapon experimentation project conducted by the United States Department of Defense from 1962 to 1973.

Sarafloxacin (INN) is a quinolone antibiotic drug, which was removed from clinical use by its manufacturer Abbott Laboratories from April 30, 2001.
Evidence Informed Policy Network (EVIPNet) is a network, sponsored by the World Health Organization (WHO), which attempts to improve public health, especially in developing countries, by coordinating the efforts of policymakers and health researchers.
The BioInitiative Report is a report on the relationship between the electromagnetic fields (EMF) associated with powerlines and wireless devices and health. It was self-published online, without peer review, on 31 August 2007, by a group "of 14 scientists, researchers, and public health policy professionals". The BioInitiative Report states that it is an examination of the controversial health risks of electromagnetic fields and radiofrequency radiation. Some updated BioInitiative material was published in a journal in an issue guest-edited by one of the members of the group, and a 2012 version of the report was released on 7 January 2013. It has been heavily criticized by independent and governmental research groups for its lack of balance.

The Joint FAO/WHO Expert Committee on Food Additives (JECFA) is an international scientific expert committee that is administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO). It has been meeting since 1956 to provide independent scientific advice pertaining to the safety evaluation of food additives. Its current scope of work now also includes the evaluation of contaminants, naturally occurring toxicants and residues of veterinary drugs in food.
WHO Expert Committee on Leprosy is constituted by World Health Organization to study the worldwide progress of Leprosy.

Fenbutrazate (INN), also known as phenbutrazate (BAN), is a psychostimulant used as an appetite suppressant under the trade names Cafilon, Filon, and Sabacid in Europe, Japan, and Hong Kong. It is a derivative of phenmetrazine and may function as a prodrug due to its similarity to phendimetrazine.
Snake antivenom is a medication made up of antibodies used to treat snake bites by venomous snakes. It is a type of antivenom.
M.K. Krishna Menon was a leading Indian gynecologist and obstetrician who was among the first to introduce an effective treatment for the state of eclampsia in pregnancy. His lytic cocktail was the popular mode of eclampsia management until it was superseded by more sophisticated regimes. He was a recipient of the Padma Shri, the fourth highest Indian civilian award.

The Central Drugs Standard Control Organisation (CDSCO) is India's national regulatory body for cosmetics, pharmaceuticals and medical devices. It serves a similar function to the European Medicines Agency of the European Union, the PMDA of Japan, the Food and Drug Administration (FDA) of the United States, and the Medicines and Healthcare products Regulatory Agency of the United Kingdom, and the State Administration for Market Regulation of China. The Indian government has announced its plan to bring all medical devices, including implants and contraceptives under a review of the Central Drugs and Standard Control Organisation (CDSCO).
Sibte Hasan Zaidi was an Indian pathologist and toxicologist. After his training in pathology at the Hammersmith Hospital in London, United Kingdom, he returned to India to continue experimental toxicology research. During his later years, he served on national and international committees, such as the World Health Organization, to advise on the harmful biological effects of industrial toxins.
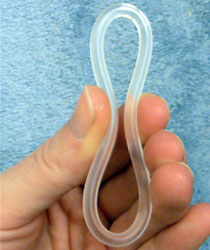
Progesterone vaginal ring, also known as progesterone-only vaginal ring, is a form of vaginal ring used for birth control when breastfeeding. Use can begin at four weeks and continue for at least up to a year following childbirth. Failure rates with usual use is about 1.5 per 100 women. It is used within the vagina with one ring lasting three months. The woman is able to place and remove the ring herself. It is sold under the brand names Progering among others.
National Immunization Technical Advisory Group (NITAG) is an advisory committee consisting of multidisciplinary groups of experts responsible for providing information to national governments that is used to make evidence-based decisions regarding vaccine and immunization policy. The majority of industrialized and some developing countries have formally established advisory committees to guide immunization policies; other countries are working towards establishment of such committees.
The Strategic Advisory Group of Experts (SAGE) is the principal advisory group to World Health Organization (WHO) for vaccines and immunization. Established in 1999 through the merging of two previous committees, notably the Scientific Advisory Group of Experts and the Global Advisory Group by Director-General of the WHO Gro Harlem Brundtland. It is charged with advising WHO on overall global policies and strategies, ranging from vaccines and biotechnology, research and development, to delivery of immunization and its linkages with other health interventions. SAGE is concerned not just with childhood vaccines and immunization, but all vaccine-preventable diseases. SAGE provide global recommendations on immunization policy and such recommendations will be further translated by advisory committee at the country level.
References
- ↑ "Vitamin Standards". Nature. 135 (3413): 516–517. March 1935. doi: 10.1038/135516a0 .