Related Research Articles
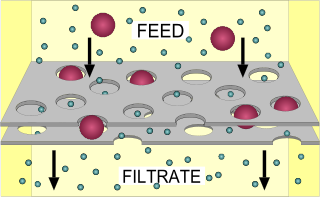
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.
A lubricant is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liquid. A supersaturated solution is in a metastable state; it may be brought to equilibrium by forcing the excess of solute to separate from the solution. The term can also be applied to a mixture of gases.

A heat exchanger is a system used to transfer heat between a source and a working fluid. Heat exchangers are used in both cooling and heating processes. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air. Another example is the heat sink, which is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant.

Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface.
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned".

Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation.

A phase-change material (PCM) is a substance which releases/absorbs sufficient energy at phase transition to provide useful heat or cooling. Generally the transition will be from one of the first two fundamental states of matter - solid and liquid - to the other. The phase transition may also be between non-classical states of matter, such as the conformity of crystals, where the material goes from conforming to one crystalline structure to conforming to another, which may be a higher or lower energy state.
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.

Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling) or a non-living substance. Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function.
Higher alkanes are alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and is not classified as dangerously flammable.

In geotechnical engineering, drilling fluid, also known as drilling mud, is used to aid the drilling of boreholes into the earth. Often used while drilling oil and natural gas wells and on exploration drilling rigs, drilling fluids are also used for much simpler boreholes, such as water wells.
In liquids, the cloud point is the temperature below which a transparent solution undergoes either a liquid-liquid phase separation to form an emulsion or a liquid-solid phase transition to form either a stable sol or a suspension that settles a precipitate. The cloud point is analogous to the 'dew point' at which a gas-liquid phase transition called condensation occurs in water vapour to form liquid water. When the temperature is below 0 °C, the dew point is called the frost point, as water vapour undergoes gas-solid phase transition called deposition, solidification, or freezing.

Clastic rocks are composed of fragments, or clasts, of pre-existing minerals and rock. A clast is a fragment of geological detritus, chunks, and smaller grains of rock broken off other rocks by physical weathering. Geologists use the term clastic to refer to sedimentary rocks and particles in sediment transport, whether in suspension or as bed load, and in sediment deposits.

In fluid mechanics, multiphase flow is the simultaneous flow of materials with two or more thermodynamic phases. Virtually all processing technologies from cavitating pumps and turbines to paper-making and the construction of plastics involve some form of multiphase flow. It is also prevalent in many natural phenomena.
An oil production plant is a facility which processes production fluids from oil wells in order to separate out key components and prepare them for export. Typical oil well production fluids are a mixture of oil, gas and produced water. An oil production plant is distinct from an oil depot, which does not have processing facilities.
Black powder is an industry name for the abrasive, reactive particulate contamination present in all gas and hydrocarbon fluid transmission lines. Black powder ranges from light brown to black, and the mineral makeup varies per production field around the world.

A defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. Strictly speaking, defoamers eliminate existing foam and anti-foamers prevent the formation of further foam. Commonly used agents are insoluble oils, polydimethylsiloxanes and other silicones, certain alcohols, stearates and glycols. The additive is used to prevent formation of foam or is added to break a foam already formed.
Oilfield scale inhibition is the process of preventing the formation of scale from blocking or hindering fluid flow through pipelines, valves, and pumps used in oil production and processing. Scale inhibitors (SIs) are a class of specialty chemicals that are used to slow or prevent scaling in water systems. Oilfield scaling is the precipitation and accumulation of insoluble crystals (salts) from a mixture of incompatible aqueous phases in oil processing systems. Scale is a common term in the oil industry used to describe solid deposits that grow over time, blocking and hindering fluid flow through pipelines, valves, pumps etc. with significant reduction in production rates and equipment damages. Scaling represents a major challenge for flow assurance in the oil and gas industry. Examples of oilfield scales are calcium carbonate (limescale), iron sulfides, barium sulfate and strontium sulfate. Scale inhibition encompasses the processes or techniques employed to treat scaling problems.
References
- ↑ Singh, Probjot; Venkatesan, Ramachandran; Fogler, H. Scott; Nagarajan, Nagi (2000). "Formation and Aging of Incipient Thin Film Wax-Oil Gel" (PDF). AIChE Journal. 46 (5): 1059–1074. doi:10.1002/aic.690460517. hdl:2027.42/34241.
- ↑ Sarica, Cem; Panacharoensawad, Ekarit (2012). "Review of Paraffin Deposition Research under Multiphase Flow Conditions". Energy & Fuels. 26 (7): 3968–3978. doi:10.1021/ef300164q.
- ↑ Panacharoensawad, Ekarit (2012). Wax Deposition Under Two-Phase Oil-Water Flowing Conditions (PhD). Tulsa University.