Related Research Articles
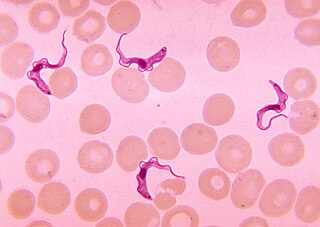
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species Trypanosoma brucei. Humans are infected by two types, Trypanosoma brucei gambiense (TbG) and Trypanosoma brucei rhodesiense (TbR). TbG causes over 98% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas.

Trypanosomatida is a group of kinetoplastid excavates distinguished by having only a single flagellum. The name is derived from the Greek trypano (borer) and soma (body) because of the corkscrew-like motion of some trypanosomatid species. All members are exclusively parasitic, found primarily in insects. A few genera have life-cycles involving a secondary host, which may be a vertebrate, invertebrate or plant. These include several species that cause major diseases in humans. Some trypanosomatida are intracellular parasites, with the important exception of Trypanosoma brucei.

Kinetoplastida is a group of flagellated protists belonging to the phylum Euglenozoa, and characterised by the presence of an organelle with a large massed DNA called kinetoplast. The organisms are commonly referred to as "kinetoplastids" or "kinetoplasts" The group includes a number of parasites responsible for serious diseases in humans and other animals, as well as various forms found in soil and aquatic environments. Their distinguishing feature, the presence of a kinetoplast, is an unusual DNA-containing granule located within the single mitochondrion associated with the base of the cell's flagellum. The kinetoplast contains many copies of the mitochondrial genome.

Tsetse, are large, biting flies that inhabit much of tropical Africa. Tsetse flies include all the species in the genus Glossina, which are placed in their own family, Glossinidae. The tsetse is an obligate parasite, which lives by feeding on the blood of vertebrate animals. Tsetse has been extensively studied, because of their role in transmitting disease. They have a prominent economic impact in sub-Saharan Africa, as the biological vectors of trypanosomes, causing human and animal trypanosomiasis.

Trypanosomiasis or trypanosomosis is the name of several diseases in vertebrates caused by parasitic protozoan trypanosomes of the genus Trypanosoma. In humans this includes African trypanosomiasis and Chagas disease. A number of other diseases occur in other animals.

Trypanosoma is a genus of kinetoplastids, a monophyletic group of unicellular parasitic flagellate protozoa. Trypanosoma is part of the phylum Sarcomastigophora. The name is derived from the Greek trypano- (borer) and soma (body) because of their corkscrew-like motion. Most trypanosomes are heteroxenous and most are transmitted via a vector. The majority of species are transmitted by blood-feeding invertebrates, but there are different mechanisms among the varying species. Some, such as Trypanosoma equiperdum, are spread by direct contact. In an invertebrate host they are generally found in the intestine, but normally occupy the bloodstream or an intracellular environment in the vertebrate host.

Melarsoprol is an arsenic-containing medication used for the treatment of sleeping sickness. It is specifically used for second-stage disease caused by Trypanosoma brucei rhodesiense when the central nervous system is involved. For Trypanosoma brucei gambiense, eflornithine or fexinidazole is usually preferred. It is effective in about 95% of people. It is given by injection into a vein.
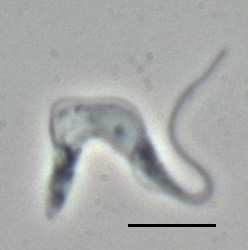
Trypanosoma brucei is a species of parasitic kinetoplastid belonging to the genus Trypanosoma that is present in sub-Saharan Africa. Unlike other protozoan parasites that normally infect blood and tissue cells, it is exclusively extracellular and inhabits the blood plasma and body fluids. It causes deadly vector-borne diseases: African trypanosomiasis or sleeping sickness in humans, and animal trypanosomiasis or nagana in cattle and horses. It is a species complex grouped into three subspecies: T. b. brucei, T. b. gambiense and T. b. rhodesiense. The first is a parasite of non-human mammals and causes nagana, while the latter two are zoonotic infecting both humans and animals and cause African trypanosomiasis.

Trypanosoma evansi is a parasitic species of excavate trypanosome in the genus Trypanosoma that is one cause of surra in animals. Discovered by Griffith Evans in 1880 at Dera Ismail Khan, it is the first known trypanosome that causes infection. It is a common parasite in India and Iran and causes acute disease in camels and horses, and chronic disease in cattle and buffalo. In Pakistan, it has been found to be the most prevalent trypanosome species in donkeys. It is now established to infect other mammals, including humans.
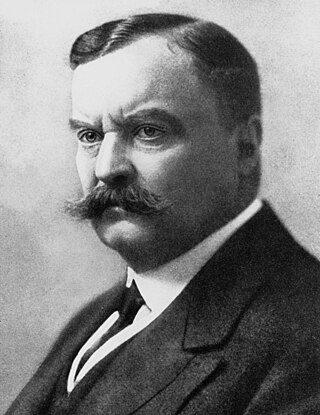
Major-General Sir David Bruce was a Scottish pathologist and microbiologist who made some of the key contributions in tropical medicine. In 1887, he discovered a bacterium, now called Brucella, that caused what was known as Malta fever. In 1894, he discovered a protozoan parasite, named Trypanosoma brucei, as the causative pathogen of nagana.
Trypanosoma suis is a species of excavate trypanosome in the genus Trypanosoma that causes one form of the surra disease in animals. It infects pigs. It does not infect humans.

Animal trypanosomiasis, also known as nagana and nagana pest, or sleeping sickness, is a disease of vertebrates. The disease is caused by trypanosomes of several species in the genus Trypanosoma such as Trypanosoma brucei. Trypanosoma vivax causes nagana mainly in West Africa, although it has spread to South America. The trypanosomes infect the blood of the vertebrate host, causing fever, weakness, and lethargy, which lead to weight loss and anemia; in some animals the disease is fatal unless treated. The trypanosomes are transmitted by tsetse flies.

Trypanosoma congolense is a species of trypanosomes and is the major pathogen responsible for the disease nagana in cattle and other animals including sheep, pigs, goats, horses and camels, dogs, as well as laboratory mice. It is the most common cause of nagana in east Africa, but is also a major cause of nagana in west Africa. This parasite is spread by tsetse flies. In its mammalian host, Trypanosoma congolense only lives in blood vessels, and causes in particular anaemia.
A Trypanosomiasis vaccine is a vaccine against trypanosomiasis. No effective vaccine currently exists, but development of a vaccine is the subject of current research.
Fexinidazole is a medication used to treat African trypanosomiasis caused by Trypanosoma brucei gambiense. It is effective against both first and second stage disease. Some evidence also supports its use in Chagas disease. It is taken by mouth.

Variant surface glycoprotein (VSG) is a ~60kDa protein which densely packs the cell surface of protozoan parasites belonging to the genus Trypanosoma. This genus is notable for their cell surface proteins. They were first isolated from Trypanosoma brucei in 1975 by George Cross. VSG allows the trypanosomatid parasites to evade the mammalian host's immune system by extensive antigenic variation. They form a 12–15 nm surface coat. VSG dimers, ~90% of all cell surface protein. It also makes up ~10% of total cell protein. For this reason, these proteins are highly immunogenic and an immune response raised against a specific VSG coat will rapidly kill trypanosomes expressing this variant. However, with each cell division there is a possibility that the progeny will switch expression to change the VSG that is being expressed. VSG has no prescribed biochemical activity.

Robert Michael Forde was Colonial Surgeon in The Gambia when in 1901, he made the first definitive observation of trypanosomes in a human being when he found them in the blood of a steamboat master on the Gambia River. In 1907 he became principal medical officer of Sierra Leone.
David Horn FRSE, is a Wellcome Trust Senior Investigator, professor of parasite molecular biology, deputy head of the Division of Biological Chemistry and Drug Discovery and deputy director of the Wellcome Trust Centre for Anti-Infectives Research in the School of Life Sciences, University of Dundee. His research is focused on antigenic variation, drug action and resistance and the application of genetic screens to African trypanosomes: parasitic protists that cause sleeping sickness or Human African Trypanosomiasis (HAT) and the livestock disease, nagana.
The Sleeping Sickness Commission was a medical project established by the British Royal Society to investigate the outbreak of African sleeping sickness or African trypanosomiasis in Africa at the turn of the 20th century. The outbreak of the disease started in 1900 in Uganda, which was at the time a protectorate of the British Empire. The initial team in 1902 consisted of Aldo Castellani and George Carmichael Low, both from the London School of Hygiene and Tropical Medicine, and Cuthbert Christy, a medical officer on duty in Bombay, India. From 1903, David Bruce of the Royal Army Medical Corps and David Nunes Nabarro of the University College Hospital took over the leadership. The commission established that species of blood protozoan called Trypanosoma brucei, named after Bruce, was the causative parasite of sleeping sickness.
Keith Roland Matthews,, , is a British cell biologist and parasitologist, currently Professor of Parasite Biology in the School of Biological Sciences at the University of Edinburgh. His research focuses on African trypanosomes, which cause human sleeping sickness and the equivalent cattle disease nagana.
References
- 1 2 3 4 5 6 "Professor Wendy Gibson". University of Bristol. Retrieved 24 December 2020.
- ↑ 50 Years of the Wellcome Trust 1936 - 1986. The Wellcome Trust. 1987. p. 139. ISBN 1-869835-01-8.
- ↑ Peacock, Lori; Ferris, Vanessa; Bailey, Mick; Gibson, Wendy (2007). "Dynamics of infection and competition between two strains of Trypanosoma brucei brucei in the tsetse fly observed using fluorescent markers". Kinetoplastid Biol Dis. 6 (4): 4. doi: 10.1186/1475-9292-6-4 . PMC 1899512 . PMID 17553128.
- ↑ Peacock, Lori; Ferris, Vanessa; Sharma, Reuben; Sunter, Jack; Bailey, Mick; Carrington, Mark; Gibson, Wendy (2011). "Identification of the meiotic life cycle stage of Trypanosoma brucei in the tsetse fly". Proceedings of the National Academy of Sciences of the United States of America. 108 (9): 3671–3676. Bibcode:2011PNAS..108.3671P. doi: 10.1073/pnas.1019423108 . PMC 3048101 . PMID 21321215.
- ↑ "Sexual reproduction in the livestock pathogen Trypanosoma congolense". UK Research and Innovation. Retrieved 25 December 2020.
- ↑ "Hidden danger from pet dogs in Africa". My Science. 29 May 2020. Retrieved 24 December 2020.
- ↑ "Trypanosoma brucei". Wellcome Sanger Institute. Retrieved 25 December 2020.
- ↑ Jackson, Andrew; Sanders, Mandy; Including Wendy Gibson, and 12 other authors (2010). "The Genome Sequence of T. brucei gambiense, Causative Agent of Chronic Human African Trypanosomiasis". PLOS Neglected Tropical Diseases. 4 (4): e658. doi: 10.1371/journal.pntd.0000658 . PMC 2854126 . PMID 20404998.
- ↑ Kelly, Steven; Ivens, Alasdair; Manna, Paul T.; Gibson, Wendy; Field, Mark C. (2014). "A draft genome for the African crocodilian trypanosome Trypanosoma grayi". Scientific Data. 1: 140024. doi: 10.1038/sdata.2014.24 . PMC 4322581 . PMID 25977781.
- ↑ Bragg, Melvyn. "in Our Time: Parasitism". BBC Radio 4. Retrieved 23 December 2020.
- ↑ "Résumé Wendy Gibson (Séance du 10 février 1996 – Mémoires couronnés) GENETIC EXCHANGE IN TRYPANOSOMES par W. GIBSON, lauréate du « Prix du Dr Albert Dubois pour la Pathologie tropicale » (période 1990-1994)". Académie royale de Médecine de Belgique. Retrieved 25 December 2020.