
Temazepam, sold under the brand name Restoril among others, is a medication of the benzodiazepine class which is generally used to treat severe or debilitating insomnia. It is taken by mouth. Temazepam is rapidly absorbed, and significant hypnotic and anxiolytic effects begin in less than 30 minutes and can last for up to eight hours. Prescriptions for hypnotics such as temazepam have seen a dramatic decrease since 2010, while anxiolytics such as alprazolam, clonazepam, and lorazepam have increased or remained stable. Temazepam and similar hypnotics, such as triazolam (Halcion) are generally reserved for severe and debilitating insomnia. They have largely been replaced by z-drugs and atypical antidepressants as first line treatment for insomnia.

Pergolide, sold under the brand name Permax and Prascend (veterinary) among others, is an ergoline-based dopamine receptor agonist used in some countries for the treatment of Parkinson's disease. Parkinson's disease is associated with reduced dopamine synthesis in the substantia nigra of the brain. Pergolide acts on many of the same receptors as dopamine to increase receptor activity.
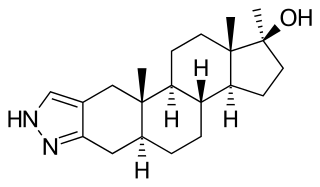
Stanozolol, sold under many brand names, is a synthetic androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Winthrop Laboratories in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given by mouth in humans or by injection into muscle in animals.

Xylazine is a structural analog of clonidine and an α2-adrenergic receptor agonist, sold under many trade names worldwide, most notably the Bayer brand name Rompun, as well as Anased, Sedazine and Chanazine.

Dextromethorphan, sold under the brand name Robitussin among others, is a cough suppressant used in many cough and cold medicines. In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropion to serve as a rapid-acting antidepressant in people with major depressive disorder.

Flunixin is a nonsteroidal anti-inflammatory drug (NSAID), analgesic, and antipyretic used in horses, cattle and pigs. It is often formulated as the meglumine salt. In the United States, it is regulated by the U.S. Food and Drug Administration (FDA), and may only be lawfully distributed by order of a licensed veterinarian. There are many trade names for the product.

Quazepam, sold under the brand name Doral among others, is a relatively long-acting benzodiazepine derivative drug developed by the Schering Corporation in the 1970s. Quazepam is used for the treatment of insomnia, including sleep induction and sleep maintenance. Quazepam induces impairment of motor function and has relatively selective hypnotic and anticonvulsant properties with considerably less overdose potential than other benzodiazepines. Quazepam is an effective hypnotic which induces and maintains sleep without disruption of the sleep architecture.
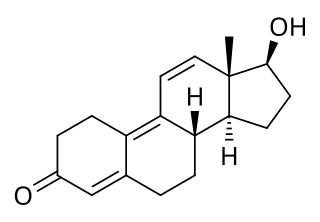
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.

Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist. It is related chemically to ketamine. Tiletamine hydrochloride exists as odorless white crystals.
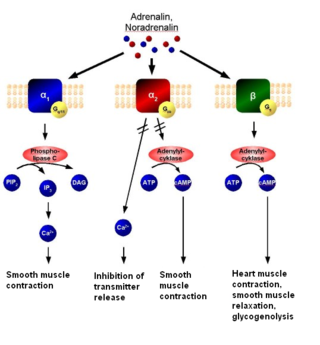
The alpha-2 (α2) adrenergic receptor is a G protein-coupled receptor (GPCR) associated with the Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-, α2B-, and α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central and peripheral nervous systems.

Florfenicol is a fluorinated synthetic analog of thiamphenicol, mainly used as a antibiotic in veterinary medicine.
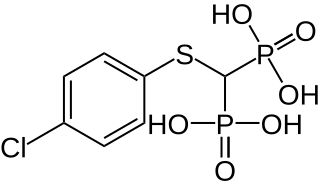
Tiludronic acid is a bisphosphonate used for treatment of Paget's disease of bone in human being medicine. It has the tradename Skelid. In veterinary medicine, tiludronic acid is used to treat navicular disease and bone spavin in horses. Its tradenames are Tildren and Equidronate. It is approved for treatment of navicular disease and distal, tarsal osteoarthritis in Europe, and was approved for treatment of navicular disease in the United States in 2014.

Isoxsuprine is a drug used as a vasodilator in humans and equines. Isoxsuprine is a β2 adrenoreceptor agonist that causes direct relaxation of uterine and vascular smooth muscle via β2 receptors.

Meclofenamic acid is a drug used for joint, muscular pain, arthritis and dysmenorrhea. It is a member of the anthranilic acid derivatives class of nonsteroidal anti-inflammatory drugs (NSAIDs) and was approved by the US FDA in 1980. Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins.

Ibafloxacin (INN) is a fluoroquinolone antibiotic drug formally approved for use in the European Union for veterinary medicine.

Cefovecin (INN) is an antibiotic of the cephalosporin class, licensed for the treatment of skin infections in cats and dogs. It is marketed by Zoetis under the trade name Convenia. It is used to treat skin infections caused by Pasteurella multocida in cats, and Staphylococcus intermedius and Streptococcus canis in dogs. The advantage of using a long-acting injectable antibiotic is that, unlike in daily administration, doses cannot be missed. Missed doses may allow partially resistant microbes to recover. The disadvantage is the presence of subtherapeutic concentrations in the weeks after the resolution of infections. This is associated with the development of resistance in microbes. It should not be used in pregnant or lactating animals or in animals with a history of allergies to penicillin or cephalosporin drugs.

Lenperone (Elanone-V) is a typical antipsychotic of the butyrophenone chemical class. It was first reported as an anti-emetic in 1974, and its use in treatment of acute schizophrenia was reported in 1975. Related early antipsychotic agents include declenperone and milenperone.
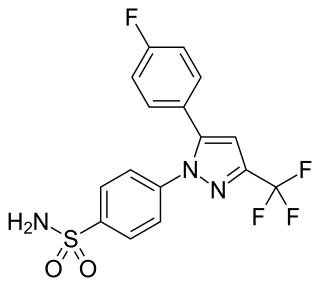
Mavacoxib is a veterinary drug used to treat pain and inflammation in dogs with degenerative joint disease. It acts as a COX-2 inhibitor.

Resocortol is a synthetic glucocorticoid corticosteroid which was never marketed.

Grapiprant, sold under the brand name Galliprant, is a small molecule drug that belongs in the piprant class. This analgesic and anti-inflammatory drug is primarily used as a pain relief for mild to moderate inflammation related to osteoarthritis in dogs. Grapiprant has been approved by the FDA's Center for Veterinary Medicine and was categorized as a non-cyclooxygenase inhibiting non-steroidal anti-inflammatory drug (NSAID) in March 2016.



















