The alkali metals are a group (column) in the periodic table consisting of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). This group lies in the s-block of the periodic table of elements as all alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour.
In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as chloramine (NClH2); see Category:Inorganic amines.
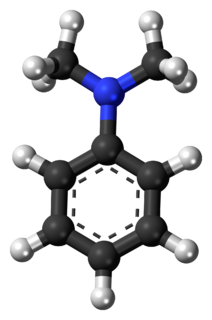
N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
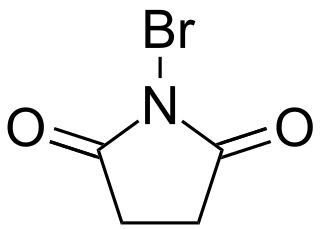
N-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.
The Bischler–Möhlau indole synthesis is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline; it is named after August Bischler and Richard Möhlau

The Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles by addition of an aniline and a ketone bearing a thioether substituent.
An azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.
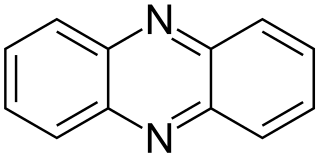
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.
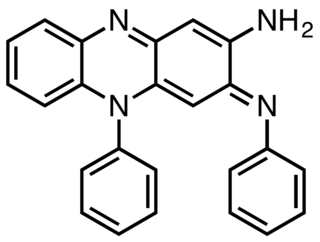
Induline is a dye of blue, bluish-red or black shades. Induline consists of a mixture of several intensely colored species, so the name is often indulines. It was one of the first synthetic dyes, discovered in 1863 by J. Dale and Heinrich Caro. The main components of induline are various substituted phenazines. Although induline no longer in use, the related dye nigrosin is still produced commercially.

The Doebner–Miller reaction is the organic reaction of an aniline with α,β-unsaturated carbonyl compounds to form quinolines.

Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. It is a bright blue species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.
In enzymology, a Methanosarcina-phenazine hydrogenase (EC 1.12.98.3) is an enzyme that catalyzes the chemical reaction
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Trans-2,3-dihydro-3-hydroxyanthranilate isomerase is an enzyme with systematic name (5S,6S)-6-amino-5-hydroxycyclohexane-1,3-diene-1-carboxyate isomerase. This enzyme catalyses the following chemical reaction

Silver nitrite is an inorganic compound with the formula AgNO2.