This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer occurring in about three people per million members of the population. It is also known as cutaneous APUDoma, primary neuroendocrine carcinoma of the skin, primary small cell carcinoma of the skin, and trabecular carcinoma of the skin. Factors involved in the development of MCC include the Merkel cell polyomavirus, a weakened immune system, and exposure to ultraviolet radiation. Merkel cell carcinoma usually arises on the head, neck, and extremities, as well as in the perianal region and on the eyelid. It is more common in people over sixty years old, Caucasian people, and males. MCC is less common in children.
Nicholas J. Vogelzang was a medical oncologist with Comprehensive Cancer Centers of Nevada (CCCN). He serves as medical director of the Research Executive Committee and Associate Chair of the Developmental Therapeutics and Genitourinary Committees for US Oncology Research. His research interests include clinical trials for genitourinary malignancies and mesothelioma.

Anaplastic thyroid cancer (ATC), also known as anaplastic thyroid carcinoma, is an aggressive form of thyroid cancer characterized by uncontrolled growth of cells in the thyroid gland. This form of cancer generally carries a very poor prognosis due to its aggressive behavior and resistance to cancer treatments. The cells of anaplastic thyroid cancer are highly abnormal and usually no longer resemble the original thyroid cells and have poor differentiation.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.

Vulvar cancer is a cancer of the vulva, the outer portion of the female genitals. It most commonly affects the labia majora. Less often, the labia minora, clitoris, or Bartholin's glands are affected. Symptoms include a lump, itchiness, changes in the skin, or bleeding from the vulva.
Bavituximab (PGN401) is a human-mouse chimeric monoclonal antibody against phosphatidylserine, which is a component of cell membranes that is exposed when a cell is transformed into solid tumor cancer cell or dies, and when cells are infected with hepatitis C. The process of cell death is highly controlled and so there usually no immune response to phosphatidylserine but when bavituximab binds to it, the conjugate appears to stimulate an immune response in humans.
Vaginal cancer is an extraordinarily rare form of cancer that develops in the tissue of the vagina. Primary vaginal cancer originates from the vaginal tissue – most frequently squamous cell carcinoma, but primary vaginal adenocarcinoma, sarcoma, and melanoma have also been reported – while secondary vaginal cancer involves the metastasis of a cancer that originated in a different part of the body. Secondary vaginal cancer is more common. Signs of vaginal cancer may include abnormal vaginal bleeding, dysuria, tenesmus, or pelvic pain, though as many as 20% of women diagnosed with vaginal cancer are asymptomatic at the time of diagnosis. Vaginal cancer occurs more frequently in women over age 50, and the mean age of diagnosis of vaginal cancer is 60 years. It often can be cured if found and treated in early stages. Surgery alone or surgery combined with pelvic radiation is typically used to treat vaginal cancer.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.

Human papillomavirus-positive oropharyngeal cancer, is a cancer of the throat caused by the human papillomavirus type 16 virus (HPV16). In the past, cancer of the oropharynx (throat) was associated with the use of alcohol or tobacco or both, but the majority of cases are now associated with the HPV virus, acquired by having oral contact with the genitals of a person who has a genital HPV infection. Risk factors include having a large number of sexual partners, a history of oral-genital sex or anal–oral sex, having a female partner with a history of either an abnormal Pap smear or cervical dysplasia, having chronic periodontitis, and, among men, younger age at first intercourse and a history of genital warts. HPV-positive OPC is considered a separate disease from HPV-negative oropharyngeal cancer.
Treatment of lung cancer refers to the use of medical therapies, such as surgery, radiation, chemotherapy, immunotherapy, percutaneous ablation, and palliative care, alone or in combination, in an attempt to cure or lessen the adverse impact of malignant neoplasms originating in lung tissue.
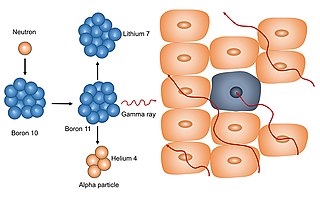
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.

Buparlisib is an experimental anti-cancer medication. It is a small molecule orally-available pan-class I phosphoinositide 3-kinase (PI3K) inhibitor. Buparlisib was under investigation as a treatment for advanced breast cancer but was abandoned due to negative results. It is still under investigation as a potential treatment for head and neck squamous cell carcinoma (HNSCC).
Sitravatinib (MGCD516) is an experimental drug for the treatment of cancer. It is a small molecule inhibitor of multiple tyrosine kinases.
Bempegaldesleukin (development code NKTR-214) is an experimental anti-cancer drug candidate. It is a PEGylated interleukin-2 (IL-2) acting as a CD122-preferential IL-2 pathway agonist designed to activate and proliferate CD8+ T cells and NK cells. It is being developed by Nektar Therapeutics.
Roy S. Herbst is an American oncologist who is the Ensign Professor of Medicine, Professor of Pharmacology, Chief of Medical Oncology, and Associate Director for Translational Research at Yale Cancer Center and Yale School of Medicine in New Haven, Connecticut.










