Related Research Articles
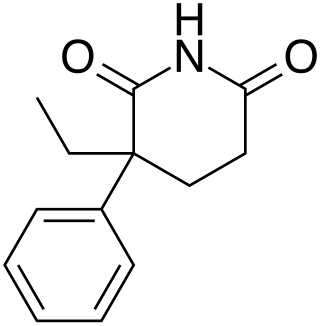
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.
Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of formaldehyde and concentrated sulfuric acid, which is dripped onto the substance being tested. The United States Department of Justice method for producing the reagent is the addition of 100 mL of concentrated (95–98%) sulfuric acid to 5 mL of 40% formaldehyde. Different compounds produce different color reactions. Methanol may be added to slow down the reaction process to allow better observation of the colour change.
A drug test is a technical analysis of a biological specimen, for example urine, hair, blood, breath, sweat, or oral fluid/saliva—to determine the presence or absence of specified parent drugs or their metabolites. Major applications of drug testing include detection of the presence of performance enhancing steroids in sport, employers and parole/probation officers screening for drugs prohibited by law and police officers testing for the presence and concentration of alcohol (ethanol) in the blood commonly referred to as BAC. BAC tests are typically administered via a breathalyzer while urinalysis is used for the vast majority of drug testing in sports and the workplace. Numerous other methods with varying degrees of accuracy, sensitivity, and detection periods exist.
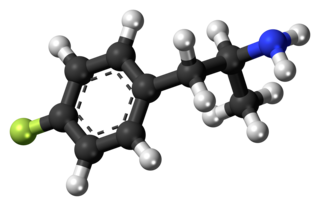
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.
Presumptive tests, in medical and forensic science, analyze a sample and establish one of the following:
- The sample is definitely not a certain substance.
- The sample probably is the substance.
The Duquenois reagent used in the Rapid Modified Duquenois–Levine test, is an established screening test for the presence of cannabis. The test was initially developed in the 1930s by the French Medical Biochemist, Pierre Duquénois (1904–1986), and was adopted in the 1950s by the United Nations as the preferred test for cannabis, and originally claimed to be specific to cannabis. After several modifications, it became known as the Duquenois–Levine test. However, in the 1960s and 70s various studies showed that the test was not specific to cannabis. In 1973, the Supreme Court of Wisconsin ruled the D–L test insufficient evidence for demonstrating that a substance was cannabis, specifically noting that the D–L tests used "are not exclusive or specific for marijuana."

Cobalt(II) thiocyanate is an inorganic compound with the formula Co(SCN)2. It is a layered coordination complex and its trihydrate Co(SCN)2(H2O)3 is used in the cobalt thiocyanate test (or Scott test) for detecting cocaine. The test has been responsible for widespread false positives and false convictions.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.
Ehrlich's reagent or Ehrlich reagent is a reagent containing p-dimethylaminobenzaldehyde (DMAB) and thus can act as an indicator to presumptively identify indoles and urobilinogen. Several Ehrlich tests use the reagent in a medical test; some are drug tests and others contribute to diagnosis of various diseases or adverse drug reactions. It is named after Nobel Prize winner Paul Ehrlich who used it to distinguish typhoid from simple diarrhoea.
The Liebermann reagent named after Hungarian chemist Leo Liebermann (1852-1926) is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of potassium nitrite and concentrated sulfuric acid. 1 g of potassium nitrite is used for every 10 mL of sulfuric acid. Potassium nitrite may also be substituted by sodium nitrite. It is used to test for cocaine, morphine, PMA and PMMA.
The Mecke reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of selenous acid and concentrated sulfuric acid, which is dripped onto the substance being tested.
The Mandelin reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of ammonium metavanadate and concentrated sulfuric acid. Its primary use is for the detection of ketamine and PMA Unlike the most common reagent test chemicals, it has a deep red colour that changes to yellow if there is no alkaloid, which occurs within about 48 hours of mixing.
Simon's reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It reacts with secondary amines like MDMA and methamphetamine to give a blue solution.
The Froehde reagent is used as a simple spot-test to presumptively identify alkaloids, especially opioids, as well as other compounds. It is composed of a mixture of molybdic acid or a molybdate salt dissolved in hot, concentrated sulfuric acid, which is then dripped onto the substance being tested.
The Dille–Koppanyi reagent is used as a simple spot-test to presumptively identify barbiturates. It is composed of a mixture of two solutions. Part A is 0.1 g of cobalt(II) acetate dihydrate dissolved in 100 ml of methanol mixed with 0.2 ml of glacial acetic acid. Part B made up of is 5% isopropylamine (v/v) in methanol. Two drops of A are dropped onto the substance followed by one drop of B and any change in colour is observed.
The Gallic acid reagent is used as a simple spot-test to presumptively identify drug precursor chemicals. It is composed of a mixture of gallic acid and concentrated sulfuric acid.
The Zimmermann reagent is used as a simple spot-test used in chromatography to presumptively identify alkaloids, especially benzodiazepines, as well as other compounds. It is therefore used in drugs testing.
Cornelis Zwikker was a Dutch scientist.

The Chen-Kao reaction is a chemical method for determining the presence of pseudoephedrine, ephedrine, and similar phenylalkylamines. The reaction is used in spot tests and is also known as Chen-Kao test. The test is often used to distinguish ephedrine, pseudoephedrine, norephedrine, cathinone and methcathinone from amphetamine and methamphetamine, which do not react with Chen’s test reagent.
References
- 1 2 O’Neal, C. L.; Crouch, D. J.; Fatah, A. A. (2000). "Validation of twelve chemical spot tests for the detection of drugs of abuse". Forensic Science International. 109 (3): 189–201. doi:10.1016/S0379-0738(99)00235-2. PMID 10725655.
- ↑ "Color Test Reagents/Kits for Preliminary Identification of Drugs of Abuse" (PDF). Law Enforcement and Corrections Standards and Testing Program. July 2000. Retrieved 2011-07-24.
- ↑ Brandenberger, Hans; Maes, Robert A. A. (1997). Analytical toxicology: for clinical, forensic, and pharmaceutical chemists. Walter de Gruyter. p. 353. ISBN 978-3-11-010731-9 . Retrieved 2012-02-28.
- ↑ de Faubert Maunder, M. J. (1975). "An improved field test for barbiturates and hydantoins with a modified cobalt(II) thiocyanate reagent". The Analyst. 100 (1197): 878–883. Bibcode:1975Ana...100..878D. doi:10.1039/an9750000878. PMID 1221881.