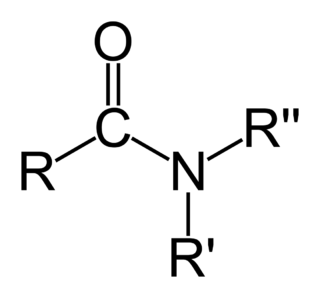
In organic chemistry, an amide ( or or, also known as an organic amide or a carboxamide, is a compound with the general formula RC NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid RC OH with the hydroxyl group –OH replaced by an amine group −NR′R″; or, equivalently, an acyl group RC − joined to an amine group.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
A carbanion is an anion in which carbon is trivalent and bears a formal negative charge.

1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.
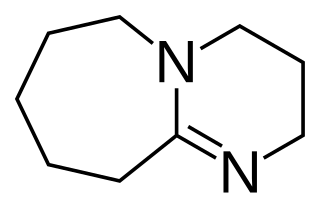
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base.

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.

Hydrogen iodide is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known.
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This colourless solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe2 substituent.
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
Triazabicyclodecene (1,5,7-triazabicyclo[4.4.0]dec-5-ene or TBD) is a commercially available bicyclic strong guanidine base (pKa = 25.98 in CH3CN and pKa = 21.00 in THF). TBD is an organic soluble base that has been used effectively for a variety of base-mediated organic transformations such as:

p-Toluenesulfonic acid (PTSA or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2 group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.
Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula RN=P(NR2)3. These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases. Another class of compounds called phosphazenes are represented with the formula [X2PN]n, where X = halide, alkoxide, amide. One example is hexachlorocyclotriphosphazene. Bis(triphenylphosphine)iminium chloride is also referred to as a phosphazene. This article focuses on those phosphazenes with the formula RN=P(NR2)3.

The oxidation of primary alcohols to carboxylic acids is an important oxidation reaction in organic chemistry.
The proton affinity of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase:
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.

In chemistry, the sulfonamide functional group (also spelled sulphonamide) is -S(=O)2-NH2, a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. The amine center is no longer basic. The S-N bond is cleaved only with difficulty. Because of the rigidity of the functional group, sulfonamides are typically crystalline. For this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.
















