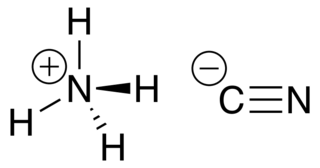In chemistry, a cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature.

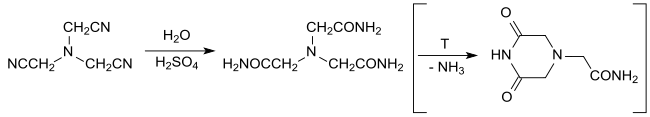
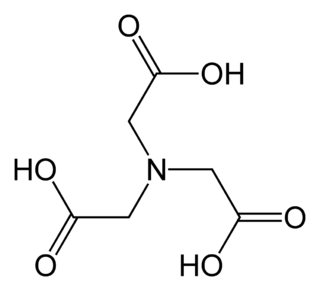
Ethylenediaminetetraacetic acid (EDTA), also called edetic acid after its own abbreviation, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, water-insoluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores. Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates, and recycled or residual materials. Processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy, and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas:

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid-liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
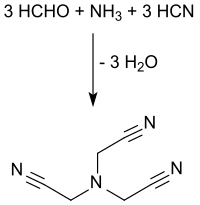
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+.
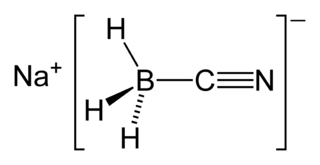
Sodium cyanoborohydride is a chemical compound with the formula Na[BH3(CN)]. It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other conventional reducing agents.

Tris(2-aminoethyl)amine is the organic compound with the formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Tris(2-aminoethyl)amine is commonly abbreviated as tren or TREN. It is used a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry.

Iminodiacetic acid is the organic compound with the formula HN(CH2CO2H)2, often abbreviated to IDA. A white solid, the compound is a dicarboxylic acid amine (the nitrogen atom forms a secondary amino group, not an imino group as the name suggests). The iminodiacetate dianion is a tridentate ligand, forming metal complexes by forming two, fused, five membered chelate rings. The proton on the nitrogen atom can be replaced by a carbon atom of a polymer to create an ion-exchange resin, such as chelex 100. Complexes of IDA and EDTA were introduced in the early 1950's by Schwarzenbach.
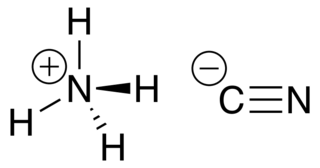
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.

2-Mercaptopyridine is an organosulfur compound with the formula HSC5H4N. This yellow crystalline solid is a derivative of pyridine. The compound and its derivatives serve primarily as acylating agents. A few of 2-mercaptopyridine’s other uses include serving as a protecting group for amines and imides as well as forming a selective reducing agent. 2-Mercaptopyridine oxidizes to 2,2’-dipyridyl disulfide.
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space.

2-Methylene glutaronitrile is a dimerization product of acrylonitrile and a starting material for di- and triamines, for the biocide 2-bromo-2-(bromomethyl)pentanedinitrile and for heterocycles, such as 3-cyanopyridine.
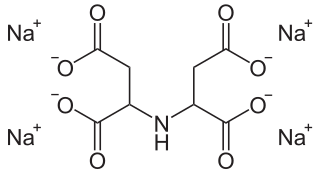
Tetrasodium iminodisuccinate is a sodium salt of iminodisuccinic acid, also referred to as N-(1,2-dicarboxyethyl)aspartic acid.

N-(2-Carboxyethyl)iminodiacetic acid or β-ADA(β-alanine diacetate) is an organic compound with the formula HO2CCH2CH2N(CH2CO2H)2. It is a white solid. The compound is classified as an aminocarboxylic acid, formally a derivative of glycine.

Trisodium N-(1-carboxylatoethyl)iminodiacetate, methylglycinediacetic acid trisodium salt (MGDA-Na3) or trisodium α-DL-alanine diacetate (α-ADA), is the trisodium anion of N-(1-carboxyethyl)iminodiacetic acid and a tetradentate complexing agent. It forms stable 1:1 chelate complexes with cations having a charge number of at least +2, e.g. the "hard water forming" cations Ca2+ or Mg2+. α-ADA is distinguished from the isomeric β-alaninediacetic acid by better biodegradability and therefore improved environmental compatibility.