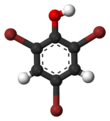
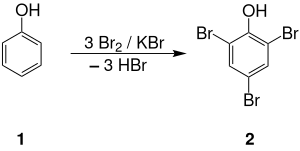
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are sometimes combined.

Creosote is a category of carbonaceous chemicals formed by the distillation of various tars and pyrolysis of plant-derived material, such as wood, or fossil fuel. They are typically used as preservatives or antiseptics.

Tylenol is a brand of medication, advertised for reducing pain, reducing fever, and relieving the symptoms of allergies, cold, cough, headache, and influenza. The active ingredient of its original flagship product is paracetamol, an analgesic and antipyretic. Like the words paracetamol and acetaminophen, the brand name Tylenol is derived from a chemical name for the compound, N-acetyl-para-aminophenol (APAP). The brand name is owned by McNeil Consumer Healthcare, a subsidiary of Kenvue.

Cork taint is a broad term referring to an off-odor and off-flavor wine fault arising from the presence of 2,4,6-trichloroanisole (TCA), a chemical compound that represents one of the strongest off-flavors, and one "generated naturally in foods/beverages", in particular wines, that considerably reduce the quality of these products.

Wood easily degrades without sufficient preservation. Apart from structural wood preservation measures, there are a number of different chemical preservatives and processes that can extend the life of wood, timber, and their associated products, including engineered wood. These generally increase the durability and resistance from being destroyed by insects or fungi.
Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The life-saving benefits of fire retardants led to their popularization. Standards for mass transit vehicles continues to increase as of 2021.
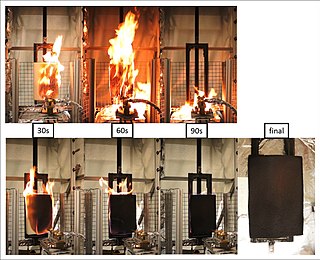
The term flame retardant subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and prevent or slow the further development of flames by a variety of different physical and chemical mechanisms. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive, while organohalogen and organophosphorus compounds can be either reactive or additive.

Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.

2,4,6-Trichloroanisole (TCA) is a chemical compound that represents one of the strongest of off-flavors, substances "generated naturally in foods/beverages [that considerably] deteriorate the quality" of such products. As of 2000, TCA was considered the primary chemical compound responsible for the phenomenon of cork taint in wines, and it has an unpleasant earthy, musty and moldy smell.
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric acid with n-butanol.
A wine fault is a sensory-associated (organoleptic) characteristic of a wine that is unpleasant, and may include elements of taste, smell, or appearance, elements that may arise from a "chemical or a microbial origin", where particular sensory experiences might arise from more than one wine fault. Wine faults may result from poor winemaking practices or storage conditions that lead to wine spoilage.
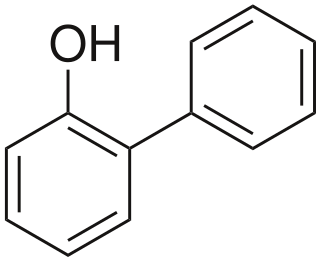
2-Phenylphenol, or o-phenylphenol, is an organic compound. In terms of structure, it is one of the monohydroxylated isomers of biphenyl. It is a white solid. It is a biocide used as a preservative with E number E231 and under the trade names Dowicide, Torsite, Fungal, Preventol, Nipacide and many others.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Octabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

2,4,6-Tribromoanisole (TBA) is a chemical compound that is a brominated derivative of anisole. It is one of the chemicals responsible for cork taint.

Decabromodiphenyl ethane is a chemical compound used as a brominated flame retardant. It was commercialised in the 1990s as an alternative for decabromodiphenyl ether, following safety concern over that compound. The two molecules are chemically very similar, which gives them a similar application profile. Decabromodiphenyl ethane is now also coming under regulatory pressure.

 [4]
[4]