
Popcorn is a variety of corn kernel which expands and puffs up when heated; the same names also refer to the foodstuff produced by the expansion.

The Maillard reaction is a chemical reaction between amino acids and reducing sugars to create melanoidins, the compounds which give browned food its distinctive flavor. Seared steaks, fried dumplings, cookies and other kinds of biscuits, breads, toasted marshmallows, and many other foods undergo this reaction. It is named after French chemist Louis Camille Maillard, who first described it in 1912 while attempting to reproduce biological protein synthesis. The reaction is a form of non-enzymatic browning which typically proceeds rapidly from around 140 to 165 °C. Many recipes call for an oven temperature high enough to ensure that a Maillard reaction occurs. At higher temperatures, caramelization and subsequently pyrolysis become more pronounced.

Diacetyl ( dy-yuh-SEE-tuhl) (IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diacetyl occurs naturally in alcoholic beverages and is added as a flavoring to some foods to impart its buttery flavor.

An aroma compound, also known as an odorant, aroma, fragrance or flavoring, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficiently volatile for transmission via the air to the olfactory system in the upper part of the nose. As examples, various fragrant fruits have diverse aroma compounds, particularly strawberries which are commercially cultivated to have appealing aromas, and contain several hundred aroma compounds.

Basmati, pronounced ['bɑːsmət̪iː], is a variety of long, slender-grained aromatic rice which is traditionally grown in the Indian subcontinent, mainly India, Pakistan, and Nepal. As of 2019, India accounted for 65% of the international trade in basmati rice, while Pakistan accounted for the remaining 35%. Many countries use domestically grown basmati rice crops; however, basmati is geographically exclusive to certain districts of India and Pakistan.
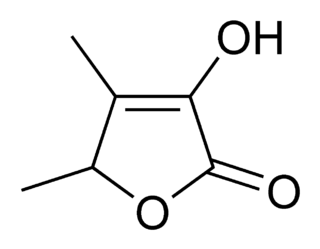
Sotolon is a lactone and an extremely powerful aroma compound, with the typical smell of fenugreek or curry at high concentrations and maple syrup, caramel, or burnt sugar at lower concentrations. Sotolon is the major aroma and flavor component of fenugreek seed and lovage, and is one of several aromatic and flavor components of artificial maple syrup. It is also present in molasses, aged rum, aged sake and white wine, flor sherry, roast tobacco, and dried fruiting bodies of the mushroom Lactarius helvus. Sotolon can pass through the body relatively unchanged, and consumption of foods high in sotolon, such as fenugreek, can impart a maple syrup aroma to one's sweat and urine. In some individuals with the genetic disorder maple syrup urine disease, it is spontaneously produced in their bodies and excreted in their urine, leading to the disease's characteristic smell.
A wine fault is a sensory-associated (organoleptic) characteristic of a wine that is unpleasant, and may include elements of taste, smell, or appearance, elements that may arise from a "chemical or a microbial origin", where particular sensory experiences might arise from more than one wine fault. Wine faults may result from poor winemaking practices or storage conditions that lead to wine spoilage.

Jasmine rice is a long-grain variety of fragrant rice. Its fragrance, reminiscent of pandan and popcorn, results from the rice plant's natural production of aroma compounds, of which 2-acetyl-1-pyrroline is the most salient. A rapid loss of aromatic intensity leads many Southeast Asians and connoisseurs to prefer each year's freshly harvested "new crop" of jasmine rice. Jasmine rice is a variety of Oryza sativa.

Maltol is a naturally occurring organic compound that is used primarily as a flavor enhancer. It is found in nature in the bark of larch trees and in the needles of pine trees, and is produced during the roasting of malt and in the baking of bread. It has the odor of caramel and is used to impart a pleasant aroma to foods and fragrances.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.
Grapefruit mercaptan is the common name for a natural organic compound found in grapefruit. It is a monoterpenoid that contains a thiol functional group. Structurally a hydroxy group of terpineol is replaced by the thiol in grapefruit mercaptan, so it also called thioterpineol. Volatile thiols typically have very strong, often unpleasant odors that can be detected by humans in very low concentrations. Grapefruit mercaptan has a very potent, but not unpleasant, odor, and it is the chemical constituent primarily responsible for the aroma of grapefruit. This characteristic aroma is a property of only the R enantiomer.

Pandanus amaryllifolius is a tropical plant in the Pandanus (screwpine) genus, which is commonly known as pandan. It has fragrant leaves which are used widely for flavouring in the cuisines of Southeast Asia and South Asia.

Oct-1-en-3-one (CH2=CHC(=O)(CH2)4CH3), also known as 1-octen-3-one, is the odorant that is responsible for the typical "metallic" smell of metals and blood coming into contact with skin. Oct-1-en-3-one has a strong metallic mushroom-like odor with an odor detection threshold of 0.03–1.12 µg/m3 and it is the main compound responsible for the "smell of metal", followed by decanal (smell: orange skin, flowery) and nonanal (smell: tallowy, fruity). Oct-1-en-3-one is the degradative reduction product of the chemical reaction of skin lipid peroxides and Fe2+. Skin lipid peroxides are formed from skin lipid by oxidation, either enzymatically by lipoxygenases or by air oxygen. Oct-1-en-3-one is a ketone analog of the alkene 1-octene.

3-Mercapto-3-methylbutan-1-ol, also known as MMB, is a common odorant found in food and cat urine. The aromas ascribed to MMB include catty, roasty, broth-like, meaty, and savory, or similar to cooked leeks.
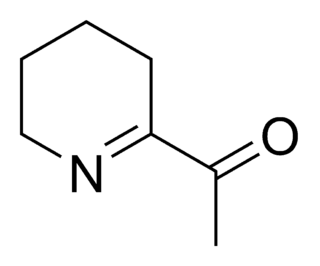
6-Acetyl-2,3,4,5-tetrahydropyridine is an aroma compound and flavor that gives baked goods such as white bread, popcorn, and tortillas their typical smell, together with its structural homolog 2-acetyl-1-pyrroline.
Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation. 1-Pyrroline is a cyclic imine, whereas 2-pyrroline and 3-pyrroline are cyclic amines.
Alkylpyrazines are chemical compounds based on pyrazine with different substitution patterns. Some alkylpyrazines are naturally occurring highly aromatic substances which often have a very low odor threshold and contribute to the taste and aroma of various foods including cocoa, baked goods, coffee and wines. Alkylpyrazines are also formed during the cooking of some foods via Maillard reactions.
(E,E)-2,4-Decadienal is an aromatic substance found in butter, cooked beef, fish, potato chips, roasted peanut, buckwheat and wheat bread crumb. In an isolated state, it smells of deep fat flavor, characteristic of chicken aroma (at 10ppm). At lower concentration, it has the odor of citrus, orange or grapefruit. It might be carcinogenic. It has been used as aroma in the EU, but use restrictions apply until the required data have been submitted.

Dimethyl trisulfide (DMTS) is an organic chemical compound and the simplest organic trisulfide, with the chemical formula CH3SSSCH3. It is a flammable liquid with a foul odor, which is detectable at levels as low as 1 part per trillion.

2-Acetylpyridine is an organic compound with the formula CH
3COC
5H
4N. It is a viscous colorless liquid that is widely used as a flavoring substance. It is found in malt and produced by the Maillard reaction and by nixtamalization. It contributes to the flavor of corn tortillas, popcorn, and beer.















