
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl groups are chemically aromatic and have equal bond lengths between carbon atoms in the ring.
A glow stick, also known as a light stick, chem light, light wand, light rod, and rave light, is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light through chemiluminescence. The light cannot be turned off and can be used only once. The used tube is then thrown away. Glow sticks are often used for recreation, such as for events, camping, outdoor exploration, and concerts. Glow sticks are also relied upon for light during military, police, fire, and emergency medical services operations. Industrial uses include marine, transportation, and mining.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

MCPA is a powerful, selective, widely used phenoxy herbicide. The pure compound is a brown-colored powder. MCPA has been extensively used in agriculture to control broad-leaf weeds as a growth regulator primarily in pasture and cereal crops field since 1945. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants. Overdose application of MCPA acts as an herbicide and results in abnormal growth.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.
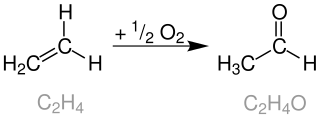
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.

Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species ClO.

Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation.

In chemistry, a phosphaalkyne is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general formula R-C≡P. Phosphaalkynes are the heavier congeners of nitriles, though, due to the similar electronegativities of phosphorus and carbon, possess reactivity patterns reminiscent of alkynes. Due to their high reactivity, phosphaalkynes are not found naturally on earth, but the simplest phosphaalkyne, phosphaethyne (H-C≡P) has been observed in the interstellar medium.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry.
Chlorinated paraffins (CPs) are complex mixtures of polychlorinated n-alkanes. The chlorination degree of CPs can vary between 30 and 70 wt%. CPs are subdivided according to their carbon chain length into short-chain CPs, medium-chain CPs and long-chain CPs. Depending on chain length and chlorine content, CPs are colorless or yellowish liquids or solids.

Butane or n-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered by the chemist Dr. Walter Snelling in 1912. It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.
4-Chlorophenol is an organic compound with the formula ClC6H4OH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.

1,1-Bis(chloromethyl)ethylene is the organic compound with the formula CH2=C(CH2Cl)2. It is a colorless liquid. Featuring two allylic chloride substituents, it is dialkylating agent.















