
A hormone is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required for the correct development of animals, plants and fungi. Due to the broad definition of a hormone, numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids, steroids, amino acid derivatives, protein or peptides, and gases.
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and through to reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.
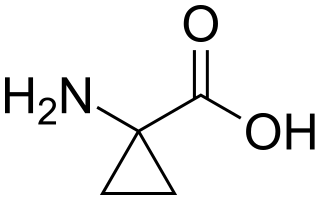
1-Aminocyclopropane-1-carboxylic acid (ACC) is a disubstituted cyclic α-amino acid in which a cyclopropane ring is fused to the Cα atom of the amino acid. It is a white solid. Many cyclopropane-substituted amino acids are known, but this one occurs naturally. Like glycine, but unlike most α-amino acids, ACC is not chiral.

Brassinosteroids are a class of polyhydroxysteroids that have been recognized as a sixth class of plant hormones and may have utility as anticancer drugs for treating endocrine-responsive cancers by inducing apoptosis of cancer cells and inhibiting cancerous growth. These brassinosteroids were first explored during the 1970s when Mitchell et al. reported promotion in stem elongation and cell division by the treatment of organic extracts of rapeseed pollen. Brassinolide was the first brassinosteroid to be isolated in 1979, when pollen from Brassica napus was shown to promote stem elongation and cell divisions, and the biologically active molecule was isolated. The yield of brassinosteroids from 230 kg of Brassica napus pollen was only 10 mg. Since their discovery, over 70 BR compounds have been isolated from plants.
Self-incompatibility (SI) is a general name for several genetic mechanisms that prevent self-fertilization in sexually reproducing organisms, and thus encourage outcrossing and allogamy. It is contrasted with separation of sexes among individuals (dioecy), and their various modes of spatial (herkogamy) and temporally (dichogamy) separation.

Phytoremediation technologies use living plants to clean up soil, air and water contaminated with hazardous contaminants. It is defined as "the use of green plants and the associated microorganisms, along with proper soil amendments and agronomic techniques to either contain, remove or render toxic environmental contaminants harmless". The term is an amalgam of the Greek phyto (plant) and Latin remedium. Although attractive for its cost, phytoremediation has not been demonstrated to redress any significant environmental challenge to the extent that contaminated space has been reclaimed.
Plant embryonic development, also plant embryogenesis is a process that occurs after the fertilization of an ovule to produce a fully developed plant embryo. This is a pertinent stage in the plant life cycle that is followed by dormancy and germination. The zygote produced after fertilization must undergo various cellular divisions and differentiations to become a mature embryo. An end stage embryo has five major components including the shoot apical meristem, hypocotyl, root meristem, root cap, and cotyledons. Unlike the embryonic development in animals, and specifically in humans, plant embryonic development results in an immature form of the plant, lacking most structures like leaves, stems, and reproductive structures. However, both plants and animals including humans, pass through a phylotypic stage that evolved independently and that causes a developmental constraint limiting morphological diversification.

Nonylphenols are a family of closely related organic compounds composed of phenol bearing a 9 carbon-tail. Nonylphenols can come in numerous structures, all of which may be considered alkylphenols. They are used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers. They are used extensively in epoxy formulation in North America but its use has been phased out in Europe. These compounds are also precursors to the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol has attracted attention due to its prevalence in the environment and its potential role as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity. The estrogenicity and biodegradation heavily depends on the branching of the nonyl sidechain. Nonylphenol has been found to act as an agonist of the GPER (GPR30).

Methyl jasmonate is a volatile organic compound used in plant defense and many diverse developmental pathways such as seed germination, root growth, flowering, fruit ripening, and senescence. Methyl jasmonate is derived from jasmonic acid and the reaction is catalyzed by S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase.

Brassinolide is a plant hormone. The first isolated brassinosteroid, it was discovered when it was shown that pollen from rapeseed could promote stem elongation and cell division. The biologically active component was isolated and named brassinolide.
Expansins are a family of closely related nonenzymatic proteins found in the plant cell wall, with important roles in plant cell growth, fruit softening, abscission, emergence of root hairs, pollen tube invasion of the stigma and style, meristem function, and other developmental processes where cell wall loosening occurs. Expansins were originally discovered as mediators of acid growth, which refers to the widespread characteristic of growing plant cell walls to expand faster at low (acidic) pH than at neutral pH. Expansins are thus linked to auxin action. They are also linked to cell enlargement and cell wall changes induced by other plant hormones such as gibberellin, cytokinin, ethylene and brassinosteroids.
Edestin, is a highly-digestible, hexameric legumin protein with six subunits, and a seed storage protein, with a molecular weight of 310 kDa. This protein is primarily found in hemp seeds. Edestin is a globular protein as opposed to fibrous protein (structural).
Peptide signaling plays a significant role in various aspects of plant growth and development and specific receptors for various peptides have been identified as being membrane-localized receptor kinases, the largest family of receptor-like molecules in plants. Signaling peptides include members of the following protein families.
Arabinogalactan-proteins (AGPs) are highly glycosylated proteins (glycoproteins) found in the cell walls of plants. Each one consists of a protein with sugar molecules attached. They are members of the wider class of hydroxyproline (Hyp)-rich cell wall glycoproteins, a large and diverse group of glycosylated wall proteins.
In plant biology, elicitors are extrinsic or foreign molecules often associated with plant pests, diseases or synergistic organisms. Elicitor molecules can attach to special receptor proteins located on plant cell membranes. These receptors are able to recognize the molecular pattern of elicitors and trigger intracellular defence signalling via the octadecanoid pathway. This response results in the enhanced synthesis of metabolites which reduce damage and increase resistance to pest, disease or environmental stress. This is an immune response called pattern triggered immunity (PTI).
Feronia, also known as FER or protein Sirene, is a recognition receptor kinase found in plants. FER plays a significant part in the plant immune system as a receptor kinase which assists in immune signaling within plants, plant growth, and plant reproduction. FER is regulated by the Rapid Alkalinization Factor (RALF). FER regulates growth in normal environments but it is most beneficial in stressful environments as it helps to initiate immune signaling. FER can also play a role in reproduction in plants by participating in the communication between the female and male cells. FER is found in and can be studied in the organism Arabidopsis thaliana.
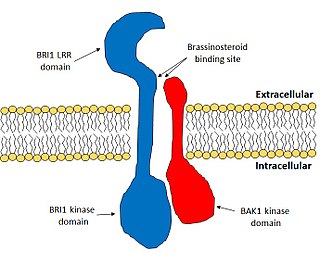
Brassinosteroid insensitive 1 (BRI1) is the major receptor of the plant hormone brassinosteroid. It plays very important roles in plant development, especially in the control of cell elongation and for the tolerance of environmental stresses. BRI1 enhances cell elongation, promotes pollen development, controls vasculature development and promotes chilling and freezing tolerance. BRI1 is one of the most well studied hormone receptors and it acts a model for the study of membrane-bound receptors in plants.
June Nasrallah is Barbara McClintock Professor in the Plant Biology Section of the School of Integrative Plant Science at Cornell University. Her research focuses on plant reproductive biology and the cell-cell interactions that underlie self-incompatibility in plants belonging to the mustard (Brassicaceae) family. She was elected to the US National Academy of Sciences in 2003 for this work and her contributions generally to our understanding of receptor-based signalling in plants.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.











