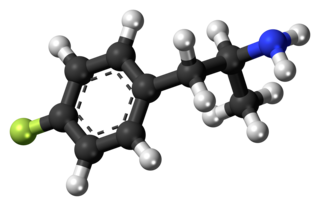
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

Lobeline is a piperidine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.
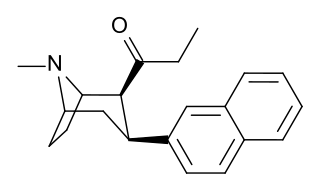
2β-Propanoyl-3β-(2-naphthyl)-tropane or WF-23 is a cocaine analogue. It is several hundred times more potent than cocaine at being a serotonin-norepinephrine-dopamine reuptake inhibitor.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.
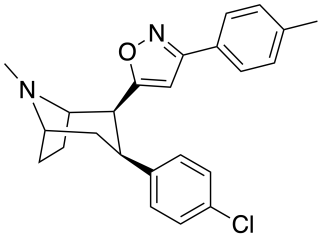
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
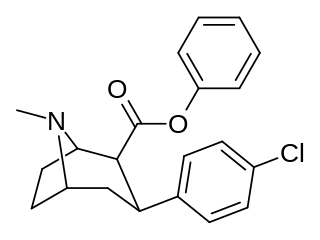
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

RTI(-4229)-112 is a synthetic stimulant drug from the phenyltropane family. In contrast to RTI-113, which is DAT selective, RTI-112 is a nonselective triple reuptake inhibitor.

(–)-2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane (RTI-4229-31) is a synthetic analog of cocaine that acts as a stimulant. Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article, RTI-31 is 64 times the strength of cocaine in terms of its potency to elicit self-administration in monkeys. WIN 35428 was 6 times weaker than RTI-31, whereas RTI-51 was 2.6 times weaker than RTI-31.

(–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane is a semi-synthetic alkaloid in the phenyltropane group of psychostimulant compounds. First publicized in the 1990s, it has not been used enough to have gained a fully established profile. RTI-51 can be expected to have properties lying somewhere in between RTI-31 and RTI-55. It has a ratio of monoamine reuptake inhibition of dopamine > serotonin > norepinephrine which is an unusual balance of effects not produced by other commonly used compounds. It has been used in its 76Br radiolabelled form to map the distribution of dopamine transporters in the brain.

4-Methylamphetamine is a stimulant and anorectic drug of the phenethylamine and amphetamine chemical classes.
3-Methylamphetamine is a stimulant drug from the amphetamine family. It is self-administered by mice to a similar extent to 4-fluoroamphetamine and has comparable properties as a monoamine releaser, although with a more balanced release of all three monoamines, as opposed to the more dopamine/noradrenaline selective fluoro analogues.

3-Fluoroamphetamine is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin. It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.

2,3-Dichlorophenylpiperazine (2,3-DCPP or DCPP) is a chemical compound from the phenylpiperazine family. It is both a precursor in the synthesis of aripiprazole and one of its metabolites. It is unclear whether 2,3-DCPP is pharmacologically active as a serotonin receptor agonist similar to its close analogue 3-chlorophenylpiperazine (mCPP), though it has been shown to act as a partial agonist of the dopamine D2 and D3 receptors.

3-Fluoroethamphetamine (3-FEA) is a stimulant drug of the amphetamine class which acts as a releasing agent of the monoamine neurotransmitters norepinephrine, dopamine and serotonin.

Difluoromethylenedioxyamphetamine is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by Daniel Trachsel and coworkers, along with the corresponding fluorinated derivatives of MDMA, MDEA, BDB and MBDB, with the aim of finding a non-neurotoxic drug able to be used as a less harmful substitute for entactogenic drugs such as MDMA. Since a major route of the normal metabolism of these compounds is scission of the methylenedioxy ring, producing neurotoxic metabolites such as alpha-methyldopamine, it was hoped that the difluoromethylenedioxy bioisostere would show increased metabolic stability and less toxicity.
RTI-83 is a phenyltropane derivative which represents a rare example of an SDRI or serotonin-dopamine reuptake inhibitor, a drug which inhibits the reuptake of the neurotransmitters serotonin and dopamine, while having little or no effect on the reuptake of the related neurotransmitter noradrenaline. With a binding affinity (Ki) of 55 nM at DAT and 28.4 nM at SERT but only 4030 nM at NET, RTI-83 has reasonable selectivity for DAT/SERT over NET

1-Aminomethyl-5-methoxyindane (AMMI), is a drug developed by a team led by David E. Nichols at Purdue University, which acts as a selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with similar affinity to DFMDA.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.



















