A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result in unexpected side effects.
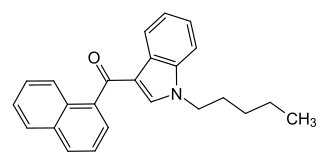
JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678 is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends".

Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids in cannabis plants attach. These novel psychoactive substances should not be confused with synthetic phytocannabinoids or synthetic endocannabinoids from which they are in many aspects distinct.

Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

4-Acetoxy-MET (4-Acetoxy-N-methyl-N-ethyltryptamine), also known as metacetin or 4-AcO-MET, is a hallucinogenic tryptamine. It is the acetate ester of 4-HO-MET, and a homologue of 4-AcO-DMT. It is a novel compound with very little history of human use. It is sometimes sold as a research chemical by online retailers.
A temporary class drug is a relatively new status for controlled drugs, which has been adopted in some jurisdictions, notably New Zealand and the United Kingdom, to attempt to bring newly synthesised designer drugs under legal control. The controlled drug legislation in these jurisdictions requires drug scheduling decisions to follow an evidence-based process, where the harms of the drug are assessed and reviewed so that an appropriate legal status can be assigned. Since many designer drugs sold in recent years have had little or no published research that could help inform such a decision, they have been widely sold as "legal highs", often for months, before sufficient evidence accumulates to justify placing them on the controlled drug schedules.
Council of the European Union decisions on designer drugs. Council of the European Union issued a set of decisions on 7 designer drugs to make them subject to control measures and criminal provisions.

MAM-2201 is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in the Netherlands and Germany in June 2011 as an ingredient in synthetic cannabis smoking blends. Like RCS-4 and AB-001, MAM-2201 thus appears to be a novel compound invented by "research chemical" suppliers specifically for grey-market recreational use. Structurally, MAM-2201 is a hybrid of two known cannabinoid compounds JWH-122 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries.

Bromazolam (XLI-268) is a triazolobenzodiazepine (TBZD) which was first synthesised in 1976, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016. It is the bromo instead of chloro analogue of alprazolam and has similar sedative and anxiolytic effects to it and other benzodiazepines. Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81nM at the α1 subtype, 0.69nM at α2 and 0.62nM at α5.

Methoxyacetylfentanyl, commonly known as MAF is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug.

Cyclopropylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. Between June and December 2017, a total of 78 cyclopropylfentanyl-related deaths with analytical confirmation in post-mortem samples were reported by various European countries. Another 115 deaths involving cyclopropylfentanyl were reported from the United States in 2017.
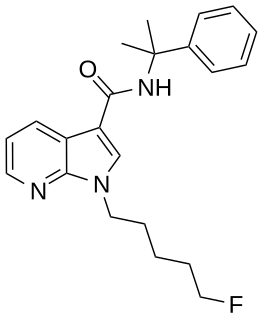
5F-CUMYL-P7AICA is a pyrrolo[2,3-b]pyridine-3-carboxamide based synthetic cannabinoid that has been sold as a designer drug. It was first identified by the EMCDDA in February 2015.

4-EA-NBOMe is a substituted amphetamine and 25-NB derivative which has been sold as a designer drug. It was first identified by a forensic laboratory in Germany in 2014, but while its analytical properties and metabolism have been studied, its pharmacology remains unknown.

TH-PVP is a substituted cathinone derivative which has been sold as a designer drug. It was first identified by a forensic laboratory in Hungary in 2015, but has subsequently been found in numerous other countries around the world including Spain, Belgium, Poland, Turkey and Brazil. Pharmacological studies in vitro showed it to inhibit reuptake and promote the release of monoamine neurotransmitters with some selectivity for serotonin, but it failed to produce stimulant effects in animals, and has a pharmacological profile more comparable to that of sedating empathogens such as MDAI and 5-Methyl-MDA.
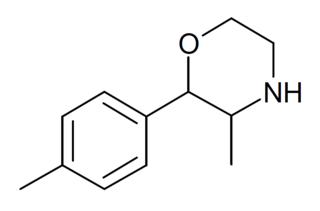
4-Methylphenmetrazine is a recreational designer drug with stimulant effects. It is a substituted phenylmorpholine derivative, closely related to better known drugs such as phenmetrazine and 3-fluorophenmetrazine. It was first identified in Slovenia in 2015, and has been shown to act as a monoamine releaser with some preference for serotonin release.

N-Ethyl-2C-B is a recreational designer drug with psychedelic effects. It was first synthesised in the 1990s, and was first identified as a new psychoactive substance in Finland in 2007. It is specifically listed as an illegal drug in Finland, and controlled under analogue provisions in a number of other jurisdictions.
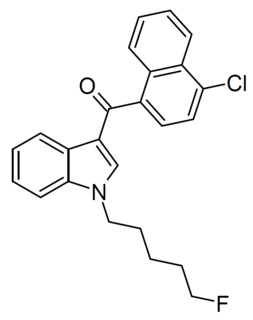
5F-JWH-398 is a recreational designer drug which is classed as a synthetic cannabinoid. It is from the naphthoylindole family, and produces cannabis-like effects. It was legally sold in New Zealand from 2012-2014 under the psychoactive substances scheme but was discontinued in May 2014 following the end of the interim approval period under the Psychoactive Substances Act 2013. Subsequently it has appeared on the illicit market around the world and was identified in Germany in May 2019.

Dipentylone is a substituted cathinone derivative with stimulant effects, which has been sold as a designer drug, first detected in Sweden in 2014.
Synthetic drugs refer to substances that are artificially modified from naturally-occurring drugs and are capable of exhibiting both therapeutic and psychoactive effects.















