
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.

Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
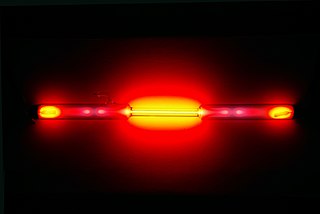
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, νέον, neuter singular form of νέος (neos), meaning new. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates.

Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant, and chemically inert transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, 103Rh. Naturally occurring rhodium is usually found as a free metal, as an alloy with similar metals, and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through which thorium and uranium slowly decay into lead and various other short-lived radioactive elements. Radon itself is the immediate decay product of radium. Its most stable isotope, 222Rn, has a half-life of only 3.8 days, making it one of the rarest elements. Since thorium and uranium are two of the most common radioactive elements on Earth, while also having three isotopes with half-lives on the order of several billion years, radon will be present on Earth long into the future despite its short half-life. The decay of radon produces many other short-lived nuclides, known as radon daughters, ending at stable isotopes of lead.

Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the International exhibition, which opened on 1 May that year.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is used in trace amounts in the fluoridation of drinking water, in toothpaste, in metallurgy, and as a flux, and is also used in pesticides and rat poison. It is a colorless or white solid that is readily soluble in water. It is a common source of fluoride in the production of pharmaceuticals and is used to prevent dental cavities.
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). The compound is primarily of interest as a component in rocket fuels, in plasmaless cleaning and etching operations in the semiconductor industry, in nuclear reactor fuel processing, and other industrial operations.

Disulfur decafluoride (S2F10) is a chemical compound discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms. S2F10 is highly toxic, with toxicity four times that of phosgene.

Aluminium fluoride refers to inorganic compounds with the formula AlF3·xH2O. They are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal. Several occur as minerals.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.

Phenyldichloroarsine, also known by its wartime name phenyl Dick and its NATO abbreviation PD, is an organic arsenical vesicant and vomiting agent developed by Germany and France for use as a chemical warfare agent during World War I. The agent is known by multiple synonyms and is technically classified as a vesicant, or blister agent.

2-Methyl-1-butanol is an organic compound with the formula CH3CH2CH(CH3)CH2OH. It is one of several isomers of amyl alcohol. A colorless liquid, it occurs naturally in trace amounts and has attracted some attention as a potential biofuel, exploiting its hydrophobic (gasoline-like) and branched structure. It is chiral.

2-Methyl-1-pentanol is an organic chemical compound. It is used as a solvent and an intermediate in the manufacture of other chemicals.

2-Methyl-2-pentanol is an organic chemical compound. It can be added to a gas chromatograph to help distinguish between branched compounds, especially alcohols. Its presence in urine can be used to test for exposure to 2-methylpentane. As with many other short-chain alcohols, 2-methyl-2-pentanol can produce intoxication and sedative effects similar to those of ethanol, though it is more irritating to mucous membranes and generally more toxic to the body.

4-Methyl-2-pentanol or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation. It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid and as a precursor to some plasticizers.

2-Methyl-3-pentanol is an organic chemical compound. It is used as a fuel.

Silver phosphate or silver orthophosphate is a light sensitive, yellow, water-insoluble chemical compound composed of silver and phosphate ions of formula Ag3PO4.

Heavy metals are generally defined as metals with relatively high densities, atomic weights, or atomic numbers. The criteria used, and whether metalloids are included, vary depending on the author and context. In metallurgy, for example, a heavy metal may be defined on the basis of density, whereas in physics the distinguishing criterion might be atomic number, while a chemist would likely be more concerned with chemical behaviour. More specific definitions have been published, but none of these have been widely accepted. The definitions surveyed in this article encompass up to 96 out of the 118 known chemical elements; only mercury, lead and bismuth meet all of them. Despite this lack of agreement, the term is widely used in science. A density of more than 5 g/cm3 is sometimes quoted as a commonly used criterion and is used in the body of this article.




















