
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).

Carnosine (beta-alanyl-L-histidine) is a dipeptide molecule, made up of the amino acids beta-alanine and histidine. It is highly concentrated in muscle and brain tissues. Carnosine was discovered by Russian chemist Vladimir Gulevich.

β-Alanine is a naturally occurring beta amino acid, which is an amino acid in which the amino group is attached to the β-carbon instead of the more usual α-carbon for alanine (α-alanine). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.

DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.

Beta-peptides (β-peptides) are peptides derived from β-amino acids, in which the amino group is attached to the β-carbon (i.e. the carbon two atoms away from the carboxylate group). The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β-peptide-based antibiotics are being explored as ways of evading antibiotic resistance. Early studies in this field were published in 1996 by the group of Dieter Seebach and that of Samuel Gellman.
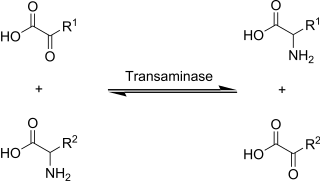
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
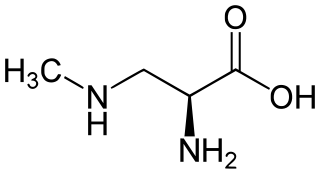
β-Methylamino-L-alanine, or BMAA, is a non-proteinogenic amino acid produced by cyanobacteria. BMAA is a neurotoxin and its potential role in various neurodegenerative disorders is the subject of scientific research.

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction

In enzymology, an aspartate 4-decarboxylase (EC 4.1.1.12) is an enzyme that catalyzes the chemical reaction
Carnosine synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a homoglutathione synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a pantoate—β-alanine ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-ureidopropionase (EC 3.5.1.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a cyanoalanine nitrilase (EC 3.5.5.4) is an enzyme that catalyzes the chemical reaction
In enzymology, a zeatin 9-aminocarboxyethyltransferase is an enzyme that catalyzes the chemical reaction

3-Aminoisobutyric acid is a product formed by the catabolism of thymine.

Cytosolic non-specific dipeptidase also known as carnosine dipeptidase 2 is an enzyme that in humans is encoded by the CNDP2 gene. This enzyme catalyses the following chemical reaction
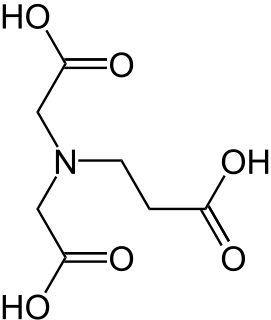
N-(2-Carboxyethyl)iminodiacetic acid or β-ADA(β-alanine diacetate) is a tetradentate complexing agent which forms stable 1:1 chelate complexes with cations having a charge number of at least +2, e.g. the "hard water forming" cations Ca2+ or Mg2+. N-(2-Carboxyethyl)iminodiacetic acid should not be confused with α-Alaninediacetic acid, also known as methylglycinediacetic acid (MGDA) or α-ADA. Alkaline earth and heavy metal complexes of both, α-ADA and β-ADA are biodegradable (in contrast to chelate complexes with conventional complexing agents such as EDTA).
Secondary amino acids are amino acids which do not contain the amino group -NH
2 but is rather a secondary amine. Secondary amino acids can be classified to cyclic acids such as proline and acyclic N-substituted amino acids.













