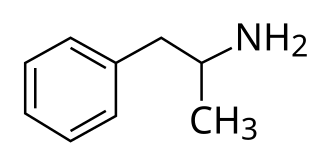
Amphetamine is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Amphetamine was discovered as a chemical in 1887 by Lazăr Edeleanu, and then as a drug in the late 1920s. It exists as two enantiomers: levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.

Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders.

Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Emsam, Selgin, among other names, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It is provided in the form of a capsule or tablet taken by mouth or orally disintegrating tablets taken on the tongue for Parkinson's disease and as a patch applied to skin for depression.
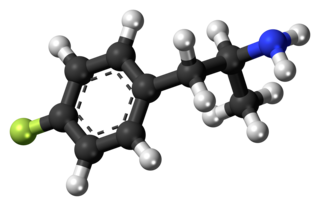
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.

Levomethamphetamine is the levorotatory (L-enantiomer) form of methamphetamine. Levomethamphetamine is a sympathomimetic vasoconstrictor that is the active ingredient in some over-the-counter (OTC) nasal decongestant inhalers in the United States.

Omigapil is a drug that was developed by Novartis and tested in clinical trials for its ability to help treat Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS). The development for PD and ALS have been terminated due to lack of benefit, but Santhera Pharmaceuticals bought the compound for development for the treatment of congenital muscular dystrophy (CMD).
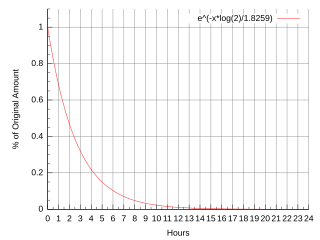
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96.7% of the time and electron capture 3.3% of the time. Both modes of decay yield stable oxygen-18.

Rasagiline is an irreversible inhibitor of monoamine oxidase-B used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.

Benzofuranylpropylaminopentane is a drug with an unusual monoamine-release potentiating mechanism of action. It can loosely be grouped with the stimulant or antidepressant drug families, but its mechanism of action is quite different.

(-)-1-Phenyl-2-propylaminopentane is a stimulant of the substituted phenethylamine class that has been derived from selegiline. When compared with selegiline and other substituted phenethylamines (-)-PPAP has a notably different mechanism of action and pharmacological effect.

Monoamine oxidase B, also known as MAO-B, is an enzyme that in humans is encoded by the MAOB gene.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).

d-Deprenyl, also known as or dextro-N-propargyl-N-methylamphetamine, is an MAO-B inhibitor that metabolizes into d-amphetamine and d-methamphetamine and is therefore also a norepinephrine–dopamine releasing agent. It is the opposite enantiomer of l-deprenyl (selegiline).

Mofegiline (MDL-72,974) is a selective, irreversible inhibitor of monoamine oxidase B (MAO-B) and semicarbazide-sensitive amine oxidase (SSAO) which was under investigation for the treatment of Parkinson's disease and Alzheimer's disease, but was never marketed.
Nifene is a high affinity, selective nicotinic α4β2* receptor partial agonist used in medical research for nicotinic acetylcholine receptors, usually in the form of nifene (18F) as a positron emission tomography (PET) radiotracer.
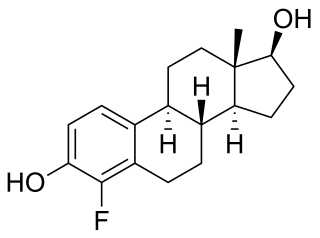
4-Fluoroestradiol (4-FE2) is a synthetic estrogen and a derivative of estradiol which was never marketed. It is specifically the 4-fluoro analogue of estradiol. 4-Fluoroestradiol has 180 ± 43% of the affinity of estradiol for the estrogen receptor of rat uterine cytosol and shows potent uterotrophic activity similar to that of estradiol in mice and rats. It has been labeled with fluorine-18 (18F) for potential use in medical imaging.


















