Olive oil is a liquid fat obtained from olives, a traditional tree crop of the Mediterranean Basin, produced by pressing whole olives and extracting the oil. It is commonly used in cooking: for frying foods or as a salad dressing. It can be found in some cosmetics, pharmaceuticals, soaps, and fuels for traditional oil lamps. It also has additional uses in some religions. The olive is one of three core food plants in Mediterranean cuisine; the other two are wheat and grapes. Olive trees have been grown around the Mediterranean since the 8th millennium BC.

The olive, botanical name Olea europaea, meaning 'European olive', is a species of small tree or shrub in the family Oleaceae, found traditionally in the Mediterranean Basin. When in shrub form, it is known as Olea europaea 'Montra', dwarf olive, or little olive. The species is cultivated in all the countries of the Mediterranean, as well as in Australia, New Zealand, North and South America and South Africa. Olea europaea is the type species for the genus Olea.

Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). Mildly acidic, it requires careful handling because it can cause chemical burns.

Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin salix for willow tree. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character.

Ferulic acid is a hydroxycinnamic acid, an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans.
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
4-Ethylphenol (4-EP) is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A white solid, it occurs as an impurity in xylenols and as such is used in the production of some commercial phenolic resins. It is also a precursor to 4-vinylphenol.

4-Ethylguaiacol, often abbreviated to 4-EG, is a phenolic compound with the molecular formula C9H12O2. It can be produced in wine and beer by Brettanomyces. It is also frequently present in bio-oil produced by pyrolysis of lignocellulosic biomass.

Hydroxytyrosol is a phenylethanoid, a type of phenolic phytochemical with antioxidant properties in vitro. In nature, hydroxytyrosol is mainly found in olives, olive leaves, and olive oil in the form of its elenolic acid ester, oleuropein. It is a constituent of red and white wines.

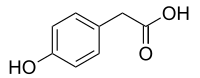
Tyrosol is a phenylethanoid, a derivative of phenethyl alcohol. It is a natural phenolic antioxidant present in a variety of natural sources. The principal source in the human diet is olive oil. It is also one of the main natural phenols in argan oil. In olive oil, tyrosol forms esters with fatty acids.

Oleuropein is a glycosylated seco-iridoid, a type of phenolic bitter compound found in green olive skin, flesh, seeds, and leaves, and argan oil. The term oleuropein is derived from the botanical name of the olive tree, Olea europaea.

Olive leaf is the leaf of the olive tree. Although olive oil is well known for its flavor and possible health benefits, the leaf and its extracts remain under preliminary research with unknown effects on human health.

EDDHA or ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) is a chelating agent. Like EDTA, it binds metal ions as a hexadentate ligand, using two amines, two phenolate centers, and two carboxylates as the six binding sites. The complexes are typically anionic. The ligand itself is a white, water soluble powder. Both the free ligand and its tetraanionic chelating agent are abbreviated EDDHA. In contrast to EDDHA, most related aminopolycarboxylic acid chelating agents feature tertiary amines and few have phenolate groups.

Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4.

The Picual, also known as Marteña or Lopereña, is an olive cultivar from Spain. Picual olives are the most commonly grown olive today for olive oil production, with production centered in the Spanish province of Jaén. Picual trees are estimated to account for 25% of all olive oil production in the world. Naturally, this varietal is very high in oil content, at 20-27% by weight.

4-Hydroxymandelic acid is a chemical compound used to synthesize atenolol. The compound typically occurs as a monohydrate.