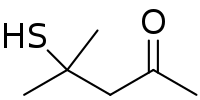
A thiol or thiol derivative is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol", where the first word deriving from Greek θεῖον (theion) meaning "sulfur".

Blue cheese is semi-soft cheese with a sharp, salty flavor. It is made with cultures of the edible mold Penicillium, giving it spots or veins throughout the cheese in shades of blue or green. It carries a distinct smell, either from the mold or from various specially cultivated bacteria such as Brevibacterium linens, which also causes foot odor and other human body odors.
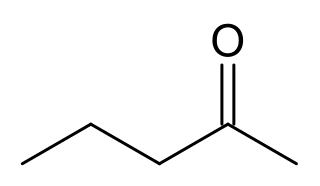
2-Pentanone or methyl propyl ketone (MPK) is a ketone and solvent of minor importance. It is comparable to methyl ethyl ketone, but has a lower solvency and is more expensive. It occurs naturally in Nicotiana tabacum (Tobacco) and blue cheese as a metabolic product of Penicillium mold growth.

3-Pentanone is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents.
Pentanone may refer to the following ketones containing five carbon atoms:

Ethionine is a non-proteinogenic amino acid structurally related to methionine, with an ethyl group in place of the methyl group.

Ethyl isopropyl ketone (2-methyl-3-pentanone) is an aliphatic ketone with used as a reagent in organic chemistry and as a solvent.
The molecular formula C5H10O may refer to:

Olfactory receptor 2M3 is a protein that in humans is encoded by the OR2M3 gene.

Olfactory receptor 51E1 is a protein that in humans is encoded by the OR51E1 gene.
Heptanone may refer to the following ketones with seven carbon atoms the formula C7H14O:
An odorizer is a device that adds an odorant to a gas. The most common type is one that adds a mercaptan liquid into natural gas distribution systems so that leaks can be readily detected. Other types have been used for carbon dioxide fire extinguishers.
Hexanone may refer to the following ketones containing six carbon atoms:

Pomarose is a high-impact captive odorant patented by Givaudan. It is a double-unsaturated ketone that does not occur in nature. Pomarose has a powerful fruity rose odor with nuances of apples, plums and raisins, which is almost entirely due to the (2E,5Z)-stereoisomer, while its (2E,5E)-isomer is barely detectable for most people. Catalyzed by traces of acids, both isomers equilibrate however quickly upon standing in glass containers.
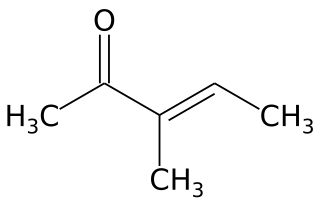
3-Methyl-3-penten-2-one is an unsaturated aliphatic ketone. It is an isomer of mesityl oxide and isomesityl oxide. It is a precursor of 3-methyl-2-pentanone and is obtained by acid-catalyzed dehydration of 4-hydroxy-3-methyl-2-pentanone. It is used as an intermediate in organic chemistry syntheses.

3-Methyl-2-pentanone is an aliphatic ketone and isomer of 2-hexanone. It is used as a solvent and as an intermediate for syntheses. Its industrial importance is low. It is produced by base-catalyzed aldol condensation of 2-butanone with acetaldehyde, forming 4-hydroxy-3-methyl-2-pentanone, which is dehydrated to 3-methyl-3-penten-2-one over an acid catalyst, followed by hydrogenation over a palladium catalyst.

4-Methylpentedrone, is a stimulant drug of the cathinone class that has been sold online as a designer drug. It is a higher homolog of 4-methylmethcathinone (mephedrone) and 4-methylbuphedrone (4-MeMABP), and the p-methyl derivative of pentedrone. It can also be viewed as the methylamino analog of pyrovalerone.

4-Methyl-α-ethylaminopentiophenone (4-MEAP) is a designer drug of the cathinone class. It is a higher homolog of 4-methylpentedrone (4-MPD) with an ethyl group in place of the methyl group. 4-MEAP has been found in samples of drugs sold as 4-MPD.

Delta-3-Tetrahydrocannabinol is a synthetic isomer of tetrahydrocannabinol, developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis plant. While the normal trans configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used.