Hypothetical types of biochemistry are forms of biochemistry agreed to be scientifically viable but not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as a solvent, and DNA or RNA to define and control their form. If life exists on other planets or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries – for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water.
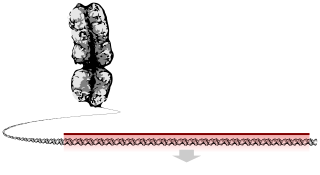
Deoxyribonucleic acid is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids. Alongside proteins, lipids and complex carbohydrates (polysaccharides), nucleic acids are one of the four major types of macromolecules that are essential for all known forms of life.
Heterochromatin is a tightly packed form of DNA or condensed DNA, which comes in multiple varieties. These varieties lie on a continuum between the two extremes of constitutive heterochromatin and facultative heterochromatin. Both play a role in the expression of genes. Because it is tightly packed, it was thought to be inaccessible to polymerases and therefore not transcribed; however, according to Volpe et al. (2002), and many other papers since, much of this DNA is in fact transcribed, but it is continuously turned over via RNA-induced transcriptional silencing (RITS). Recent studies with electron microscopy and OsO4 staining reveal that the dense packing is not due to the chromatin.

Deoxycytidine is a deoxyribonucleoside, a component of deoxyribonucleic acid. It is similar to the ribonucleoside cytidine, but with one hydroxyl group removed from the C2' position.
Methylcytosine may refer to:

DNA synthesis is the natural or artificial creation of deoxyribonucleic acid (DNA) molecules. DNA is a macromolecule made up of nucleotide units, which are linked by covalent bonds and hydrogen bonds, in a repeating structure. DNA synthesis occurs when these nucleotide units are joined to form DNA; this can occur artificially or naturally. Nucleotide units are made up of a nitrogenous base, pentose sugar (deoxyribose) and phosphate group. Each unit is joined when a covalent bond forms between its phosphate group and the pentose sugar of the next nucleotide, forming a sugar-phosphate backbone. DNA is a complementary, double stranded structure as specific base pairing occurs naturally when hydrogen bonds form between the nucleotide bases.

The Association of Zoos and Aquariums (AZA), originally the American Association of Zoological Parks and Aquariums, is an American 501(c)(3) nonprofit organization founded in 1924 and dedicated to the advancement of zoos and public aquariums in the areas of conservation, education, science, and recreation. AZA is headquartered in Silver Spring, Maryland, and accredits zoos. There were 238 accredited facilities as of 2019, primarily in the US, and also a handful in eleven other countries.

Luc Montagnier was a French virologist and joint recipient, with Françoise Barré-Sinoussi and Harald zur Hausen, of the 2008 Nobel Prize in Physiology or Medicine for his discovery of the human immunodeficiency virus (HIV). He worked as a researcher at the Pasteur Institute in Paris and as a full-time professor at Shanghai Jiao Tong University in China.
Pyrosequencing is a method of DNA sequencing based on the "sequencing by synthesis" principle, in which the sequencing is performed by detecting the nucleotide incorporated by a DNA polymerase. Pyrosequencing relies on light detection based on a chain reaction when pyrophosphate is released. Hence, the name pyrosequencing.

Azacitidine, sold under the brand name Vidaza among others, is a medication used for the treatment of myelodysplastic syndrome, myeloid leukemia, and juvenile myelomonocytic leukemia. It is a chemical analog of cytidine, a nucleoside in DNA and RNA. Azacitidine and its deoxy derivative, decitabine were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.

Decitabine, sold under the brand name Dacogen among others, acts as a nucleic acid synthesis inhibitor. It is a medication for the treatment of myelodysplastic syndromes, a class of conditions where certain blood cells are dysfunctional, and for acute myeloid leukemia (AML). Chemically, it is a cytidine analog.
Synthetic genomics is a nascent field of synthetic biology that uses aspects of genetic modification on pre-existing life forms, or artificial gene synthesis to create new DNA or entire lifeforms.

Isoguanine or 2-hydroxyadenine is a purine base that is an isomer of guanine. It is a product of oxidative damage to DNA and has been shown to cause mutation. It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.
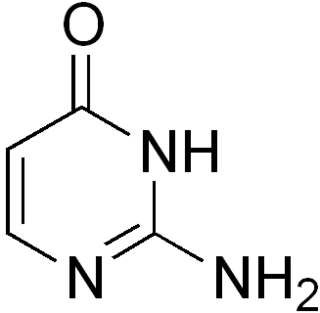
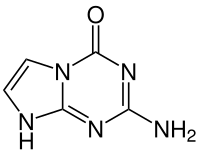
Isocytosine or 2-aminouracil is a pyrimidine base that is an isomer of cytosine. It is used in combination with isoguanine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA. In particular, it is used as a nucleobase of hachimoji RNA.
Artificially Expanded Genetic Information System (AEGIS) is a synthetic DNA analog experiment that uses some unnatural base pairs from the laboratories of the Foundation for Applied Molecular Evolution in Gainesville, Florida. AEGIS is a NASA-funded project to try to understand how extraterrestrial life may have developed.

The Spiralia are a morphologically diverse clade of protostome animals, including within their number the molluscs, annelids, platyhelminths and other taxa. The term Spiralia is applied to those phyla that exhibit canonical spiral cleavage, a pattern of early development found in most members of the Lophotrochozoa.

Hachimoji DNA is a synthetic nucleic acid analog that uses four synthetic nucleotides in addition to the four present in the natural nucleic acids, DNA and RNA. This leads to four allowed base pairs: two unnatural base pairs formed by the synthetic nucleobases in addition to the two normal pairs. Hachimoji bases have been demonstrated in both DNA and RNA analogs, using deoxyribose and ribose respectively as the backbone sugar.

1-Methylcytosine is a methylated form of the DNA base cytosine.

6-Amino-5-nitropyridin-2-one or 6-amino-5-nitro-2(1H)-pyridinone is a pyridine base. It is used as a nucleobase of hachimoji DNA, in which it pairs with 5-aza-7-deazaguanine.
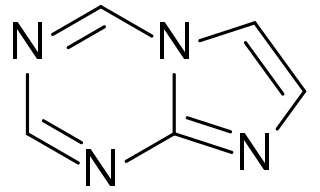
5-Aza-7-deazapurine or imidazo[1,2-a][1,3,5]triazine is a heterocyclic aromatic organic compound that consists of a s-triazine ring fused to an imidazole ring. It is an isostere and isomer of purine. However, in 5-aza-7-deazapurine, N-9 of five-membered ring does not bond with hydrogen. So 5-aza-7-deazapurine derivatives must have an exocyclic substituent with a double bond to bind a sugar residue. 5-Aza-7-deazapurine nucleosides may have an oxo, thioxo, or a imine group.