Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Musk is a class of aromatic substances commonly used as base notes in perfumery. They include glandular secretions from animals such as the musk deer, numerous plants emitting similar fragrances, and artificial substances with similar odors. Musk was a name originally given to a substance with a strong odor obtained from a gland of the musk deer. The substance has been used as a popular perfume fixative since ancient times and is one of the most expensive animal products in the world. The name originates from the Late Greek μόσχος 'moskhos', from Persian mushk and Sanskrit मुष्क muṣka derived from Proto-Indo-European noun múh₂s meaning "mouse". The deer gland was thought to resemble a scrotum. It is applied to various plants and animals of similar smell and has come to encompass a wide variety of aromatic substances with similar odors, despite their often differing chemical structures and molecular shapes.

Leopold Ružička was a Croatian-Swiss scientist and joint winner of the 1939 Nobel Prize in Chemistry "for his work on polymethylenes and higher terpenes" "including the first chemical synthesis of male sex hormones." He worked most of his life in Switzerland, and received eight doctorates honoris causa in science, medicine, and law; seven prizes and medals; and twenty-four honorary memberships in chemical, biochemical, and other scientific societies.
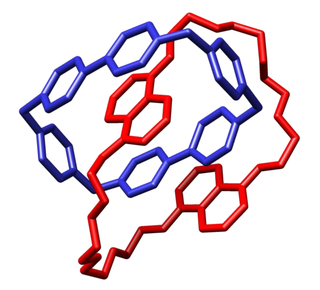
In macromolecular chemistry, a catenane is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.

Civetone is a macrocyclic ketone and the main odorous constituent of civet oil. It is a pheromone sourced from the African civet. It has a strong musky odor that becomes pleasant at extreme dilutions. Civetone is closely related to muscone, the principal odoriferous compound found in musk; the structure of both compounds was elucidated by Leopold Ružička. Today, civetone can be synthesized from precursor chemicals found in palm oil.

Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.

Tetrapropylammonium perruthenate (TPAP or TPAPR) is the chemical compound described by the formula N(C3H7)4RuO4. Sometimes known as the Ley–Griffith reagent, this ruthenium compound is used as a reagent in organic synthesis. This salt consists of the tetrapropylammonium cation and the perruthenate anion, RuO−4.

Muscone is an organic compound that is the primary contributor to the odor of musk.

Rhizoxin is an antimitotic agent with anti-tumor activity. It is isolated from a pathogenic plant fungus which causes rice seedling blight.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Synthetic musks are a class of synthetic aroma compounds to emulate the scent of deer musk and other animal musks. Synthetic musks have a clean, smooth and sweet scent lacking the fecal notes of animal musks. They are used as flavorings and fixatives in cosmetics, detergents, perfumes and foods, supplying the base note of many perfume formulas. Most musk fragrance used in perfumery today is synthetic.

Philip Kraft is a German organic chemist. Since 1996 he has been employed by Givaudan, a leading Flavor and Fragrance company, where he designs captive odorants for use in perfumes. He has lectured at the University of Bern, the University of Zurich, and the ETH Zurich.

Anthony Gerard Martin Barrett FRS, FMedSci is a British chemist, and Sir Derek Barton Professor of Synthesis, Glaxo Professor of Organic Chemistry at Imperial College London. He is Director of the Wolfson Centre for Organic Chemistry in Medical Science. He was elected a fellow of the Royal Society in 1999 and Academy of Medical Sciences in 2003. He obtained a BSc as well as PhD from Imperial College London in 1973 and 1975 respectively.

Laurolactam is an organic compound from the group of macrocyclic lactams. Laurolactam is mainly used as a monomer in engineering plastics, such as nylon-12 and copolyamides.

The preorbital gland is a paired exocrine gland found in many species of hoofed animals, which is homologous to the lacrimal gland found in humans. These glands are trenchlike slits of dark blue to black, nearly bare skin extending from the medial canthus of each eye. They are lined by a combination of sebaceous and sudoriferous glands, and they produce secretions which contain pheromones and other semiochemical compounds. Ungulates frequently deposit these secretions on twigs and grass as a means of communication with other animals.
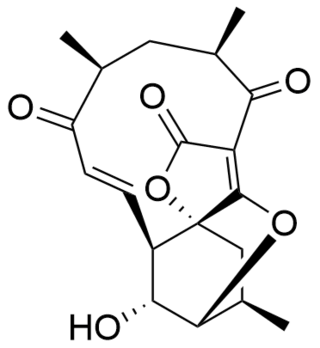
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.
Sasanka Chandra Bhattacharyya (1918–2013) was an Indian natural product chemist and the director of Bose Institute, Kolkata. He was known for his studies on structures and configurations of terpenoids and synthesis of Vetiver Oil and natural musk. He was the vice-president of the Indian National Science Academy and was an elected fellow of the academy as well as the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1962, for his contributions to chemical sciences.
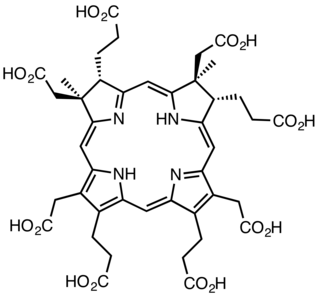
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis.

Corey–Nicolaou macrolactonization is a named reaction of organic chemistry, for the synthesis of lactones from hydroxy acids, found in 1974. The reaction should take place in a polar aprotic solvent with mild conditions, with the use of 2,2'-Dipyridyldisulfide and triphenylphosphine.

















