Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
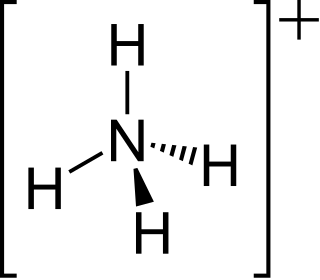
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

Potassium bromide (KBr) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion. Potassium bromide is used as a veterinary drug, in antiepileptic medication for dogs.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver, which is signaled by grey to black or purplish coloration in some samples. AgCl occurs naturally as a mineral chlorargyrite.

1,2,4-Trihydroxyanthraquinone, commonly called purpurin, is an anthraquinone. It is a naturally occurring red/yellow dye. It is formally derived from 9,10-anthraquinone by replacement of three hydrogen atoms by hydroxyl (OH) groups.

Solvent Violet 13, also known as D&C Violet No.2, oil violet, Solvent Blue 90, Alizarine Violet 3B, Alizurol Purple, Duranol Brilliant Violet TG, Ahcoquinone Blue IR base, Quinizarin Blue, Disperse Blue 72, and C.I. 60725, is a synthetic anthraquinone dye with bright bluish violet hue. It is a solid insoluble in water and soluble in acetone, toluene, and benzene. Its chemical formula is C21H15NO3, and its structure is 1-hydroxy-4-(p-tolylamino)anthraquinone, or 1-hydroxy-4-[(4-methylphenyl)amino]-9,10-anthracenedione or 1-hydroxy-4-(4-methylanilino)anthraquinone.
Zinc hydroxide Zn(OH)2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal).

For the parent molecule 9,10-anthraquinone, see anthraquinone
In enzymology, an alizarin 2-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction

2-Ethylanthraquinone is an organic compound that is a derivative of anthraquinone. This pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).
The molecular formula C14H10O2 (molar mass: 210.23 g/mol, exact mass: 210.0681 u) may refer to:
A trihydroxyanthraquinone or trihydroxyanthracenedione is any of several isomeric organic compounds with formula C
14H
8O
5, formally derived from anthraquinone by replacing three hydrogen atoms by hydroxyl groups. They include several historically important dyes. The isomers may differ in the parent anthraquinone isomer and/or of the three hydroxyl groups.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
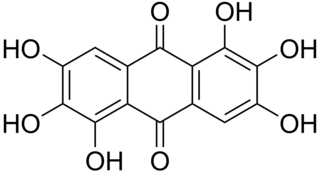
Rufigallol or 1,2,3,5,6,7-hexahydroxy-9,10-anthraquinone is an organic compound with formula C
14O
8H
8, which can be viewed as a derivative of anthraquinone through the replacement of six hydrogen atoms (H) by hydroxyl groups (OH).

Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone. That parent is sometimes simply called quinone, and this is the only hydroxy derivative of it.
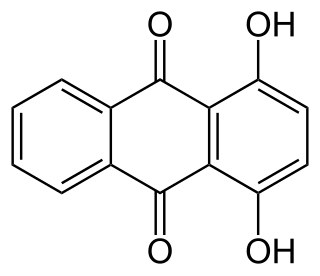
A hydroxyanthraquinone (formula: C14H7O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

Disperse Red 60, or 1-amino-4-hydroxy-2-phenoxyanthraquinone, is a popular disperse dye of the anthraquinone family of dyes. It is a dark red solid that is insoluble in water but soluble in dichloromethane.













