 | |
| Names | |
|---|---|
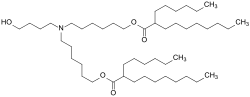
| Preferred IUPAC name [(4-Hydroxybutyl)azanediyl]di(hexane-6,1-diyl) bis(2-hexyldecanoate) | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C48H95NO5 | |
| Molar mass | 766.290 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
ALC-0315 ([(4-hydroxybutyl)azanediyl]di(hexane-6,1-diyl) bis(2-hexyldecanoate)) is a synthetic lipid. A colorless oily material, it has attracted attention as a component of the SARS-CoV-2 vaccine, BNT162b2, from BioNTech and Pfizer. Specifically, it is one of four components that form lipid nanoparticles (LNPs), which encapsulate and protect the otherwise fragile mRNA that is the active ingredient in these drugs. [1] [2] These nanoparticles promote the uptake of therapeutically effective nucleic acids such as oligonucleotides or mRNA both in vitro and in vivo . [3] [4]
Contents
Below physiological pH, ALC-0315 becomes protonated at the nitrogen atom, yielding an ammonium cation that is attracted to the messenger RNA (mRNA), which is anionic. [5]