Arachidonate 15-lipoxygenase type II is an enzyme that in humans is encoded by the ALOX15B gene. [5] [6] ALOX15B, also known as 15-lipoxygenase-2 (15-LO-2 or 15-LOX-2), is distinguished from its related oxygenase, ALOX15 or 15-lipoxygenase-1.
Arachidonate 15-lipoxygenase type II is an enzyme that in humans is encoded by the ALOX15B gene. [5] [6] ALOX15B, also known as 15-lipoxygenase-2 (15-LO-2 or 15-LOX-2), is distinguished from its related oxygenase, ALOX15 or 15-lipoxygenase-1.
This gene encodes a member of the lipoxygenase family of structurally related nonheme iron dioxygenases involved in the production of fatty acid hydroperoxides. 15-LOX-2 has 38-39% amino acid sequence identity to human 15-LOX-1 and 12-lipoxygenase and 44% amino acid sequence identity to human 5-lipoxygenase. [5] 15-LOX-2 converts arachidonic acid almost exclusively to the S stereoisomer of 15-Hydroperoxyicosatetraenoic acid which is commonly reduced to the S stereoisomer 15-Hydroxyeicosatetraenoic acid by ubiquitous cellular peroxidases; it metabolizes linoleic acid less effectively, converting this fatty acid to the S stereoisomer of 13-hydroperoxyoctadecadienoic acid which is likewise rapidly reduced to the S stereoisomer of 13-Hydroxyoctadecadienoic acid. [5] The ALOX15B gene is located in a cluster of related genes and a pseudogene that spans approximately 100 kilobases on the short arm of chromosome 17. Alternatively spliced transcript variants encoding different isoforms have been described. [6]

Reed–Sternberg cells are distinctive, giant cells found with light microscopy in biopsies from individuals with Hodgkin lymphoma. They are usually derived from B lymphocytes, classically considered crippled germinal center B cells. In the vast majority of cases, the immunoglobulin genes of Reed-Sternberg cells have undergone both V(D)J recombination and somatic hypermutation, establishing an origin from a germinal center or postgerminal center B cell. Despite having the genetic signature of a B cell, the Reed-Sternberg cells of classical Hodgkin lymphoma fail to express most B-cell–specific genes, including the immunoglobulin genes. The cause of this wholesale reprogramming of gene expression has yet to be fully explained. It presumably is the result of widespread epigenetic changes of uncertain etiology, but is partly a consequence of so-called “crippling” mutations acquired during somatic hypermutation. Seen against a sea of B cells, they give the tissue a moth-eaten appearance.

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.

Arachidonate 5-lipoxygenase-activating protein also known as 5-lipoxygenase activating protein, or FLAP, is a protein that in humans is encoded by the ALOX5AP gene.
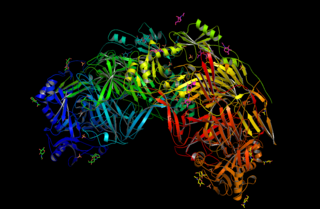
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the LOX gene. It catalyzes the conversion of lysine molecules into highly reactive aldehydes that form cross-links in extracellular matrix proteins. Its inhibition can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.
Arachidonate 5-lipoxygenase inhibitors are compounds that slow or stop the action of the arachidonate 5-lipoxygenase enzyme, which is responsible for the production of inflammatory leukotrienes. The overproduction of leukotrienes is a major cause of inflammation in asthma, allergic rhinitis, and osteoarthritis.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

Homeobox protein Nkx-3.1, also known as NKX3-1, NKX3, BAPX2, NKX3A and NKX3.1 is a protein that in humans is encoded by the NKX3-1 gene located on chromosome 8p. NKX3-1 is a prostatic tumor suppressor gene.

Leukotriene B4 receptor 2, also known as BLT2, BLT2 receptor, and BLTR2, is an Integral membrane protein that is encoded by the LTB4R2 gene in humans and the Ltbr2 gene in mice.

5-Hydroxyeicosatetraenoic acid is an eicosanoid, i.e. a metabolite of arachidonic acid. It is produced by diverse cell types in humans and other animal species. These cells may then metabolize the formed 5(S)-HETE to 5-oxo-eicosatetraenoic acid (5-oxo-ETE), 5(S),15(S)-dihydroxyeicosatetraenoic acid, or 5-oxo-15-hydroxyeicosatetraenoic acid.

Coactosin-like protein is a protein that in humans is encoded by the COTL1 gene.

Arachidonate 12-lipoxygenase, 12R type, also known as ALOX12B, 12R-LOX, and arachidonate lipoxygenase 3, is a lipoxygenase-type enzyme composed of 701 amino acids and encoded by the ALOX12B gene. The gene is located on chromosome 17 at position 13.1 where it forms a cluster with two other lipoxygenases, ALOXE3 and ALOX15B. Among the human lipoxygenases, ALOX12B is most closely related in amino acid sequence to ALOXE3

Epidermis-type lipoxygenase 3 is a member of the lipoxygenase family of enzymes; in humans, it is encoded by the ALOXE3 gene. This gene is located on chromosome 17 at position 13.1 where it forms a cluster with two other lipoxygenases, ALOX12B and ALOX15B. Among the human lipoxygenases, ALOXE3 is most closely related in amino acid sequence to ALOX12B. ALOXE3, ALOX12B, and ALOX15B are often classified as epidermal lipoxygenases, in distinction to the other three human lipoxygenases, because they were initially defined as being highly or even exclusively expressed and functioning in skin. The epidermis-type lipoxygenases are now regarded as a distinct subclass within the multigene family of mammalian lipoxygenases with mouse Aloxe3 being the ortholog to human ALOXE3, mouse Alox12b being the ortholog to human ALOX12B, and mouse Alox8 being the ortholog to human ALOX15B [supplied by OMIM]. ALOX12B and ALOXE3 in humans, Alox12b and Aloxe3 in mice, and comparable orthologs in other in other species are proposed to act sequentially in a multistep metabolic pathway that forms products that are structurally critical for creating and maintaining the skin's water barrier function.
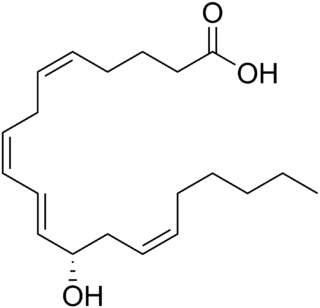
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid, made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.
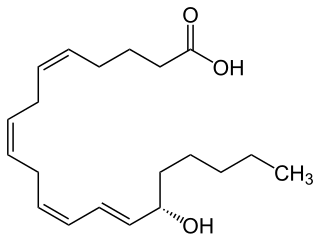
15-Hydroxyeicosatetraenoic acid is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid. This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5S,15S-dihydroxy-eicosatetraenoic acid, 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15 -HETE, a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15 -HETE and 15 -HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.
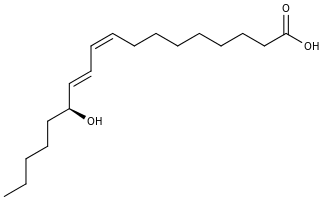
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid. The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid. The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid and 13(R)-hydroxy-9E,11E-octadecadienoic acid. Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.
Specialized pro-resolving mediators are a large and growing class of cell signaling molecules formed in cells by the metabolism of polyunsaturated fatty acids (PUFA) by one or a combination of lipoxygenase, cyclooxygenase, and cytochrome P450 monooxygenase enzymes. Pre-clinical studies, primarily in animal models and human tissues, implicate SPM in orchestrating the resolution of inflammation. Prominent members include the resolvins and protectins.