Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes. The amount and spread of fallout is a product of the size of the weapon and the altitude at which it is detonated. Fallout may get entrained with the products of a pyrocumulus cloud and fall as black rain. This radioactive dust, usually consisting of fission products mixed with bystanding atoms that are neutron-activated by exposure, is a form of radioactive contamination.

The sievert is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation.
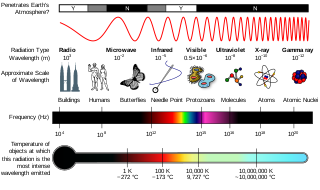
Ionizing radiation (US) (or ionising radiation [UK]), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.
The gray is the unit of ionizing radiation dose in the International System of Units (SI), defined as the absorption of one joule of radiation energy per kilogram of matter.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.
The roentgen equivalent man (rem) is a CGS unit of equivalent dose, effective dose, and committed dose, which are dose measures used to estimate potential health effects of low levels of ionizing radiation on the human body.

Radiation hormesis is the hypothesis that low doses of ionizing radiation are beneficial, stimulating the activation of repair mechanisms that protect against disease, that are not activated in absence of ionizing radiation. The reserve repair mechanisms are hypothesized to be sufficiently effective when stimulated as to not only cancel the detrimental effects of ionizing radiation but also inhibit disease not related to radiation exposure. It has been a mainstream concept since at least 2009.
The rad is a unit of absorbed radiation dose, defined as 1 rad = 0.01 Gy = 0.01 J/kg. It was originally defined in CGS units in 1953 as the dose causing 100 ergs of energy to be absorbed by one gram of matter. The material absorbing the radiation can be human tissue, air, water, or any other substance.
Radioresistance is the level of ionizing radiation that organisms are able to withstand.

A radiation burn is a damage to the skin or other biological tissue and organs as an effect of radiation. The radiation types of greatest concern are thermal radiation, radio frequency energy, ultraviolet light and ionizing radiation.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the effects of ionizing radiation on living things, in particular health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.
Radiation-induced cognitive decline describes the possible correlation between radiation therapy and cognitive impairment. Radiation therapy is used mainly in the treatment of cancer. Radiation therapy can be used to cure care or shrink tumors that are interfering with quality of life. Sometimes radiation therapy is used alone; other times it is used in conjunction with chemotherapy and surgery. For people with brain tumors, radiation can be an effective treatment because chemotherapy is often less effective due to the blood–brain barrier. Unfortunately for some patients, as time passes, people who received radiation therapy may begin experiencing deficits in their learning, memory, and spatial information processing abilities. The learning, memory, and spatial information processing abilities are dependent on proper hippocampus functionality. Therefore, any hippocampus dysfunction will result in deficits in learning, memory, and spatial information processing ability.

A gamma ray, also known as gamma radiation (symbol
γ
), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz), each gamma ray imparts the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.

The medical effects of the atomic bomb upon humans can be put into the four categories below, with the effects of larger thermonuclear weapons producing blast and thermal effects so large that there would be a negligible number of survivors close enough to the center of the blast who would experience prompt/acute radiation effects, which were observed after the 16 kiloton yield Hiroshima bomb, due to its relatively low yield:

Recognized effects of higher acute radiation doses are described in more detail in the article on radiation poisoning. Although the International System of Units (SI) defines the sievert (Sv) as the unit of radiation dose equivalent, chronic radiation levels and standards are still often given in units of millirems (mrem), where 1 mrem equals 1/1,000 of a rem and 1 rem equals 0.01 Sv. Light radiation sickness begins at about 50–100 rad.
The committed dose in radiological protection is a measure of the stochastic health risk due to an intake of radioactive material into the human body. Stochastic in this context is defined as the probability of cancer induction and genetic damage, due to low levels of radiation. The SI unit of measure is the sievert.
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation. Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no evidence of this has been observed.
Chronic radiation syndrome (CRS), or chronic radiation enteritis, is a constellation of health effects of radiation that occur after months or years of chronic exposure to high amounts of radiation. Chronic radiation syndrome develops with a speed and severity proportional to the radiation dose received, unlike radiation-induced cancer. It is distinct from acute radiation syndrome, in that it occurs at dose rates low enough to permit natural repair mechanisms to compete with the radiation damage during the exposure period. Dose rates high enough to cause the acute form are fatal long before onset of the chronic form. The lower threshold for chronic radiation syndrome is between 0.7 and 1.5 Gy, at dose rates above 0.1 Gy/yr. This condition is primarily known from the Kyshtym disaster, where 66 cases were diagnosed. It has received little mention in Western literature; but see the ICRP’s 2012 Statement.
Radiation exposure is a measure of the ionization of air due to ionizing radiation from photons. It is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air. As of 2007, "medical radiation exposure" was defined by the International Commission on Radiological Protection as exposure incurred by people as part of their own medical or dental diagnosis or treatment; by persons, other than those occupationally exposed, knowingly, while voluntarily helping in the support and comfort of patients; and by volunteers in a programme of biomedical research involving their exposure. Common medical tests and treatments involving radiation include X-rays, CT scans, mammography, lung ventilation and perfusion scans, bone scans, cardiac perfusion scan, angiography, radiation therapy, and more. Each type of test carries its own amount of radiation exposure. There are two general categories of adverse health effects caused by radiation exposure: deterministic effects and stochastic effects. Deterministic effects are due to the killing/malfunction of cells following high doses; and stochastic effects involve either cancer development in exposed individuals caused by mutation of somatic cells, or heritable disease in their offspring from mutation of reproductive (germ) cells.