Related Research Articles

Fanconi anaemia (FA) is a rare, AR, genetic disease resulting in impaired response to DNA damage in the FA/BRCA pathway. Although it is a very rare disorder, study of this and other bone marrow failure syndromes has improved scientific understanding of the mechanisms of normal bone marrow function and development of cancer. Among those affected, the majority develop cancer, most often acute myelogenous leukemia (AML), MDS, and liver tumors. 90% develop aplastic anemia by age 40. About 60–75% have congenital defects, commonly short stature, abnormalities of the skin, arms, head, eyes, kidneys, and ears, and developmental disabilities. Around 75% have some form of endocrine problem, with varying degrees of severity. 60% of FA is FANC-A, 16q24.3, which has later onset bone marrow failure.
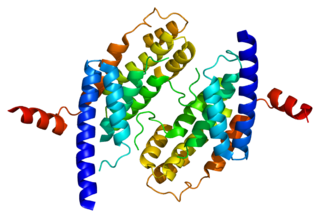
Telomeric repeat-binding factor 2 is a protein that is present at telomeres throughout the cell cycle. It is also known as TERF2, TRF2, and TRBF2, and is encoded in humans by the TERF2 gene. It is a component of the shelterin nucleoprotein complex and a second negative regulator of telomere length, playing a key role in the protective activity of telomeres. It was first reported in 1997 in the lab of Titia de Lange, where a DNA sequence similar, but not identical, to TERF1 was discovered, with respect to the Myb-domain. De Lange isolated the new Myb-containing protein sequence and called it TERF2.

Telomeric repeat-binding factor 1 is a protein that in humans is encoded by the TERF1 gene.

Fanconi anemia group D2 protein is a protein that in humans is encoded by the FANCD2 gene. The Fanconi anemia complementation group (FANC) currently includes FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, FANCM, FANCN and FANCO.
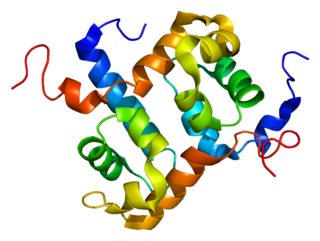
ERCC4 is a protein designated as DNA repair endonuclease XPF that in humans is encoded by the ERCC4 gene. Together with ERCC1, ERCC4 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.

Fanconi anemia group F protein is a protein that in humans is encoded by the FANCF gene.

E3 ubiquitin-protein ligase FANCL is an enzyme that in humans is encoded by the FANCL gene.

Fanconi anemia group B protein is a protein that in humans is encoded by the FANCB gene.

Fanconi anemia, complementation group I (FANCI) also known as KIAA1794, is a protein which in humans is encoded by the FANCI gene. Mutations in the FANCI gene are known to cause Fanconi anemia.

DNA cross-link repair 1B protein is a protein that in humans is encoded by the DCLRE1B gene.

Fanconi anemia, complementation group M, also known as FANCM is a human gene. It is an emerging target in cancer therapy, in particular cancers with specific genetic deficiencies.

SLX4 is a protein involved in DNA repair, where it has important roles in the final steps of homologous recombination. Mutations in the gene are associated with the disease Fanconi anemia.
Telomere-binding proteins function to bind telomeric DNA in various species. In particular, telomere-binding protein refers to TTAGGG repeat binding factor-1 (TERF1) and TTAGGG repeat binding factor-2 (TERF2). Telomere sequences in humans are composed of TTAGGG sequences which provide protection and replication of chromosome ends to prevent degradation. Telomere-binding proteins can generate a T-loop to protect chromosome ends. TRFs are double-stranded proteins which are known to induce bending, looping, and pairing of DNA which aids in the formation of T-loops. They directly bind to TTAGGG repeat sequence in the DNA. There are also subtelomeric regions present for regulation. However, in humans, there are six subunits forming a complex known as shelterin.
Simon Joseph Boulton is a British scientist who has made important contributions to the understanding of DNA repair and the treatment of cancer resulting from DNA damage. He currently occupies the position of Senior Scientist and group leader of the DSB Repair Metabolism Laboratory at the Francis Crick Institute, London. He is also an honorary Professor at University College London.
Shelterin is a protein complex known to protect telomeres in many eukaryotes from DNA repair mechanisms, as well as to regulate telomerase activity. In mammals and other vertebrates, telomeric DNA consists of repeating double-stranded 5'-TTAGGG-3' (G-strand) sequences along with the 3'-AATCCC-5' (C-strand) complement, ending with a 50-400 nucleotide 3' (G-strand) overhang. Much of the final double-stranded portion of the telomere forms a T-loop (Telomere-loop) that is invaded by the 3' (G-strand) overhang to form a small D-loop (Displacement-loop).

Titia de Lange is the Director of the Anderson Center for Cancer Research, the Leon Hess professor and the head of Laboratory Cell Biology and Genetics at Rockefeller University.

FANCD2/FANCI-associated nuclease 1 (KIAA1018) is an enzyme that in humans is encoded by the FAN1 gene. It is a structure dependent endonuclease. It is thought to play an important role in the Fanconi Anemia (FA) pathway.

SLX4 interacting protein is a protein that in humans is encoded by the SLX4IP gene.

Ketan Jayakrishna Patel is a British-Kenyan scientist who is Director of the MRC Weatherall Institute of Molecular Medicine and the MRC Molecular Haematology Unit at the University of Oxford. Until 2020 he was a tenured principal investigator at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB).

Orlando David Schärer is a Swiss chemist and biologist researching DNA repair, genomic integrity, and cancer biology. Schärer has taught biology, chemistry and pharmacology at various university levels on three continents. He is a distinguished professor at the Ulsan National Institute of Science and Technology (UNIST) and the associate director of the IBS Center for Genomic Integrity located in Ulsan, South Korea. He leads the three interdisciplinary research teams in the Chemical & Cancer Biology Branch of the center and specifically heads the Cancer Therapeutics Mechanisms Section.
References
- ↑ "Agata Smogorzewska, M.D., Ph.D. Appointed to JBC Editorial Board | Tri-Institutional MD-PhD Program". mdphd.weill.cornell.edu. Retrieved 2019-10-03.
- ↑ "Mice engineered with rare kidney disease shed light on how cells repair broken DNA". ScienceDaily. Retrieved 2019-10-03.
- 1 2 3 4 5 6 7 8 9 "Agata Smogorzewska". Our Scientists. Retrieved 2019-09-07.
- 1 2 3 4 5 6 "Smogorzewska Laboratory". Smogorzewska Laboratory. Retrieved 2019-09-07.
- 1 2 3 "Agata Smogorzewska, MD, PhD". Pershing Square Foundation. Retrieved 2019-09-07.
- ↑ "News | Member Update". www.asbmb.org. Retrieved 2019-09-07.
- ↑ "Genome Integrity Discussion Group February 2017". New York Academy of Sciences. Archived from the original on 2020-09-28.
- ↑ "JCI Editor's update: Plan S, JCI Scholars, recent reviews, and more". The American Society for Clinical Investigation. Archived from the original on 2020-08-08.
- ↑ "Poster Listing | ASHG 2012 Annual Meeting". www.ashg.org. Retrieved 2019-09-07.