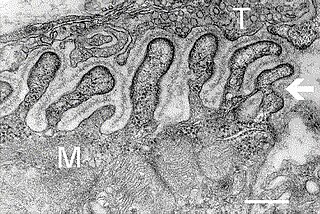
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.
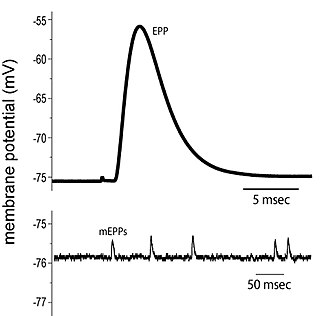
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.

A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.

Phoneutria nigriventer is a species of medically significant spider in the family Ctenidae, found in the Southern Cone of South America. Along with other members of the genus, they are often referred to as Brazilian wandering spiders.
omega-Grammotoxin SIA (ω-grammotoxin SIA) is a protein toxin that inhibits P, Q, and N voltage-gated calcium channels (Ca2+ channels) in neurons.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.
AG 489 is a component of the venom produced by Agelenopsis aperta, a North American funnel web spider. It inhibits the ligand gated ion channel TRPV1 through a pore blocking mechanism.

Spider toxins are a family of proteins produced by spiders which function as neurotoxins. The mechanism of many spider toxins is through blockage of calcium channels.

Agelenopsis aperta, also known as the desert grass spider or funnel-web spider, is a species of spider belonging to the family Agelenidae and the genus Agelenopsis. It is found in dry and arid regions across the southern United States and into northwestern Mexico. Their body is about 13–18 mm long and they have relatively long legs in order to run after their prey. Desert grass spiders can withstand very low temperatures even though they do not cold harden. It constructs the characteristic funnel-shaped webs in crevices where the funnel will fit, where they wait in the tube for prey which they can run after using their long legs. They often hunt for their prey at night.
Pompilidotoxins (PMTXs) are toxic substances that can only be found in the venom of several solitary wasps. This kind of wasp uses their venom to offensively capture prey and is relatively harmless to humans. This is in stark contrast to social insects that defend themselves and their colonies with their venom.
Birtoxin is a neurotoxin from the venom of the South African Spitting scorpion. By changing sodium channel activation, the toxin promotes spontaneous and repetitive firing much like pyrethroid insecticides do
delta-Palutoxins (δ-palutoxins) consist of a homologous group of four insect-specific toxins from the venom of the spider Pireneitega luctuosa. They show a high toxicity against Spodoptera litura larvae by inhibiting sodium channels, leading to strong paralytic activity and eventually to the death of the insect.
Raventoxins are neurotoxins from the venom of the spider Macrothele raveni.
Hainantoxins (HNTX) are neurotoxins from the venom of the Chinese bird spider Haplopelma hainanum. Hainantoxins specifically inhibit tetrodotoxin-sensitive Voltage-gated sodium channels, thereby causing blockage of neuromuscular transmission and paralysis. Currently, 13 different hainantoxins are known, but only HNTX-I, -II, -III, -IV and -V have been investigated in detail.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.
Agelenin, also called U1-agatoxin-Aop1a, is an antagonist of the presynaptic P-type calcium channel in insects. This neurotoxic peptide consists of 35 amino acids and can be isolated from the venom of the spider Allagelena opulenta.
GTx1-15 is a toxin from the Chilean tarantula venom that acts as both a voltage-gated calcium channel blocker and a voltage-gated sodium channel blocker.
Protoxin-II, also known as ProTx-II, PT-II or β/ω-TRTX-Tp2a, is a neurotoxin that inhibits certain voltage-gated calcium and voltage-gated sodium channels. This toxin is a 30-residue disulfide-rich peptide that has unusually high affinity and selectivity toward the human Nav1.7. channel.
Protoxin-I, also known as ProTx-I, or Beta/omega-theraphotoxin-Tp1a, is a 35-amino-acid peptide neurotoxin extracted from the venom of the tarantula Thrixopelma pruriens. Protoxin-I belongs to the inhibitory cystine knot (ICK) family of peptide toxins, which have been known to potently inhibit voltage-gated ion channels. Protoxin-I selectively blocks low voltage threshold T-type calcium channels, voltage gated sodium channels and the nociceptor cation channel TRPA1. Due to its unique ability to bind to TRPA1, Protoxin-I has been implicated as a valuable pharmacological reagent with potential applications in clinical contexts with regards to pain and inflammation







