DNA topoisomerases are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues in DNA arise due to the intertwined nature of its double-helical structure, which, for example, can lead to overwinding of the DNA duplex during DNA replication and transcription. If left unchanged, this torsion would eventually stop the DNA or RNA polymerases involved in these processes from continuing along the DNA helix. A second topological challenge results from the linking or tangling of DNA during replication. Left unresolved, links between replicated DNA will impede cell division. The DNA topoisomerases prevent and correct these types of topological problems. They do this by binding to DNA and cutting the sugar-phosphate backbone of either one or both of the DNA strands. This transient break allows the DNA to be untangled or unwound, and, at the end of these processes, the DNA backbone is resealed. Since the overall chemical composition and connectivity of the DNA do not change, the DNA substrate and product are chemical isomers, differing only in their topology.
DNA gyrase, or simply gyrase, is an enzyme within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA-polymerase or by helicase in front of the progressing replication fork. It is the only known enzyme to actively contribute negative supercoiling to DNA, while it also is capable of relaxing positive supercoils. It does so by looping the template to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in bacteria, whose single circular DNA is cut by DNA gyrase and the two ends are then twisted around each other to form supercoils. Gyrase is also found in eukaryotic plastids: it has been found in the apicoplast of the malarial parasite Plasmodium falciparum and in chloroplasts of several plants. Bacterial DNA gyrase is the target of many antibiotics, including nalidixic acid, novobiocin, albicidin, and ciprofloxacin.
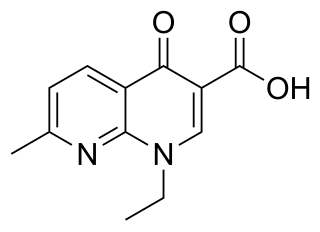
Nalidixic acid is the first of the synthetic quinolone antibiotics.
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used as chemotherapy for cancer.

Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.
Phytotoxins are substances that are poisonous or toxic to the growth of plants. Phytotoxic substances may result from human activity, as with herbicides, or they may be produced by plants, by microorganisms, or by naturally occurring chemical reactions.

Rhizoxin is an antimitotic agent with anti-tumor activity. It is isolated from a pathogenic plant fungus which causes rice seedling blight.
Xanthomonas albilineans is a species of bacteria. It causes leaf scald in sugarcane and is the source of the phytoxin and antibiotic albicidin.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:
In enzymology, glutamate racemase is an enzyme that catalyzes the chemical reaction
A nucleic acid inhibitor is a type of antibacterial that acts by inhibiting the production of nucleic acids. There are two major classes: DNA inhibitors and RNA inhibitors. The antifungal flucytosine acts in a similar manner.

Betaenone B, like other betaenones, is a secondary metabolite isolated from the fungus Pleospora betae, a plant pathogen. Its phytotoxic properties have been shown to cause sugar beet leaf spots, which is characterized by black, pycnidia containing, concentric circles eventually leading to necrosis of the leaf tissue. Of the seven phytotoxins isolated in fungal leaf spots from sugar beet, betaenone B showed the least amount of phytotoxicity showing only 8% inhibition of growth while betaenone A and C showed 73% and 89% growth inhibition, respectively. Betaenone B is therefore not considered toxic to the plant, but will produce leaf spots when present in high concentrations (0.33 μg/μL). While the mechanism of action of betaenone B has yet to be elucidated, betaenone C has been shown to inhibit RNA and protein synthesis. Most of the major work on betaenone B, including the initial structure elucidation of betaenone A, B and C as well as the partial elucidation mechanism of biosynthesis, was presented in three short papers published between 1983–88. The compounds were found to inhibit a variety of protein kinases signifying a possible role in cancer treatment.
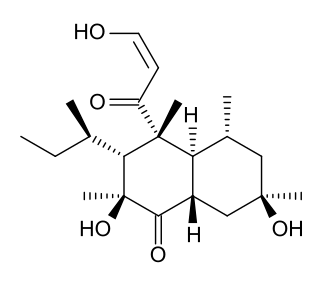
Betaenone C, like other betaenones, is a secondary metabolite isolated from the fungus Pleospora betae, a plant pathogen. Of the seven phytotoxins isolated in fungal leaf spots from sugar beet, it showed 89% growth inhibition. Betaenone C has been shown to act by inhibiting RNA and protein synthesis.

Neisseria gonorrhoeae, the bacterium that causes the sexually transmitted infection gonorrhea, has developed antibiotic resistance to many antibiotics. The bacteria was first identified in 1879.
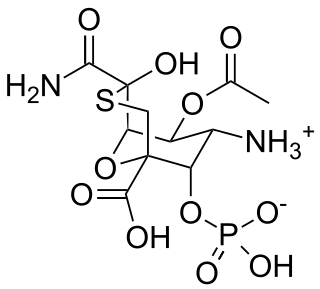
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.
Elaiomycin is an antimicrobial chemical compound, classified as an azoxyalkene, which was first isolated from Streptomyces in 1954. A laboratory synthesis of elaiomycin was reported in 1977.
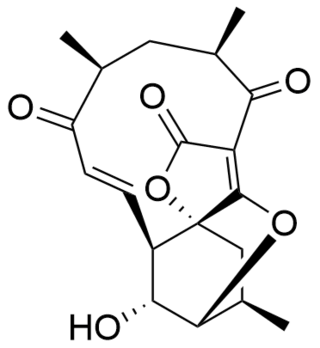
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB, thereby depleting the biosynthesis of p-aminobenzoate.
LeDock is a proprietary flexible molecular docking software designed for the purpose of docking ligands with protein targets. It is available for Linux, macOS, and Windows.

The arylomycins are a class of antibiotics initially isolated from a soil sample obtained in Cape Coast, Ghana. In this initial isolation, two families of closely related arylomycins, A and B, were identified. The family of glycosylated arylomycin C lipopeptides were subsequently isolated from a Streptomyces culture in a screen for inhibitors of bacterial signal peptidase. The initially isolated arylomycins have a limited spectrum of activity against Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pneumoniae. The only activity against Gram-negative bacteria was seen in strains with a compromised outer membrane.










