Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however the term applies to any phytochemicals that are induced by microbial infection.

Maltol is a naturally occurring organic compound that is used primarily as a flavor enhancer. It is found in nature in the bark of larch trees and in the needles of pine trees, and is produced during the roasting of malt and in the baking of bread. It has the odor of caramel and is used to impart a pleasant aroma to foods and fragrances.

In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes [V(CN)6]3− and [V2Cl9]3− are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively.
Vanadocene dichloride is an organometallic complex with formula (η5-C5H5)2VCl2 (commonly abbreviated as Cp2VCl2). It is a structural analogue of titanocene dichloride but with vanadium(IV) instead of titanium(IV). This compound has one unpaired electron, hence Cp2VCl2 is paramagnetic. Vanadocene dichloride is a suitable precursor for variety of bis(cyclopentadienyl)vanadium(IV) compounds.

Vanadium(III) chloride is the inorganic compound with the formula VCl3 which forms the hexahydrate, [VCl2(H2O)4]Cl·2H2O. This hygroscopic purple salt is a common precursor to other vanadium(III) complexes.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Vanadium compounds are compounds formed by the element vanadium (V). The chemistry of vanadium is noteworthy for the accessibility of the four adjacent oxidation states 2–5, whereas the chemistry of the other group 5 elements, niobium and tantalum, are somewhat more limited to the +5 oxidation state. In aqueous solution, vanadium forms metal aquo complexes of which the colours are lilac [V(H2O)6]2+, green [V(H2O)6]3+, blue [VO(H2O)5]2+, yellow-orange oxides [VO(H2O)5]3+, the formula for which depends on pH. Vanadium(II) compounds are reducing agents, and vanadium(V) compounds are oxidizing agents. Vanadium(IV) compounds often exist as vanadyl derivatives, which contain the VO2+ center.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.

AS-8112 is a synthetic compound that acts as a selective antagonist at the dopamine receptor subtypes D2 and D3, and the serotonin receptor 5-HT3. It has potent antiemetic effects in animal studies and has been investigated for potential medical use.

George Guy Dodson FRS FMedSci, was a British biochemist who specialised in protein crystallography at the University of York.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

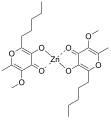
Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water.
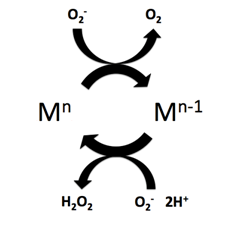
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme. SOD mimetics effectively convert the superoxide anion, a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase. Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases. SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.

Dihexa, also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is an oligopeptide drug derived from angiotensin IV that binds with high affinity to hepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal models of Alzheimer's disease-like mental impairment. In an assay of neurotrophic activity, Dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor.

R7 is a small-molecule flavonoid and orally active, potent, and selective agonist of the tropomyosin receptor kinase B (TrkB) – the main signaling receptor for the neurotrophin brain-derived neurotrophic factor (BDNF) – which is under development for the treatment of Alzheimer's disease. It is a structural modification and prodrug of tropoflavin (7,8-DHF) with improved potency and pharmacokinetics, namely oral bioavailability and duration.
Zinc L-carnosine, often simply called zinc carnosine, and also known as polaprezinc, is a mucosal protective chelate compound of zinc and L-carnosine invented by Hamari Chemicals, Ltd. It is a quadridentate 1:1 complex of a polymeric nature. Although it contains 23% zinc and 77% L-carnosine by mass, zinc carnosine is a molecule and not a mixture of zinc and L-carnosine.

Chaetochromin, also known as 4548-G05, is an orally active, small-molecule, selective agonist of the insulin receptor. It has potent and long-lasting antidiabetic activity in vivo in mice. The drug may represent a novel potential therapeutic agent for the treatment of diabetes which is more convenient and tolerable to administer than injected insulin. It was discovered in 1981 in Chaetomium gracile fungi, and its interaction with the insulin receptor was identified in 2014.

BNN-20, also known as 17β-spiro-(androst-5-en-17,2'-oxiran)-3β-ol, is a synthetic neurosteroid, "microneurotrophin", and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA, TrkB, and p75NTR, receptors for the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as for DHEA and DHEA sulfate (DHEA-S). The drug has been suggested as a potential novel treatment for Parkinson's disease and other conditions.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.
Neurotrophin mimetics are small molecules or peptide like molecules that can modulate the action of the neurotrophin receptor. One of the main causes of neurodegeneration involves changes in the expression of neurotrophins (NTs) and/or their receptors. Indeed, these imbalances or changes in their activity, lead to neuronal damage resulting in neurological and neurodegenerative conditions. The therapeutic properties of neurotrophins attracted the focus of many researchers during the years, but the poor pharmacokinetic properties, such as reduced bioavailability and low metabolic stability, the hyperalgesia, the inability to penetrate the blood–brain barrier and the short half-lives render the large neurotrophin proteins not suitable to be implemented as drugs.