Related Research Articles

A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device.

Aluminium gallium arsenide (AlxGa1−xAs) is a semiconductor material with very nearly the same lattice constant as GaAs, but a larger bandgap. The x in the formula above is a number between 0 and 1 - this indicates an arbitrary alloy between GaAs and AlAs.

A laser diode is a semiconductor device similar to a light-emitting diode in which a diode pumped directly with electrical current can create lasing conditions at the diode's junction.

Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.
Wide-bandgap semiconductors are semiconductor materials which have a larger band gap than conventional semiconductors. Conventional semiconductors like silicon have a bandgap in the range of 0.6 – 1.5 electronvolt (eV), whereas wide-bandgap materials have bandgaps in the range above 2 eV. Generally, wide-bandgap semiconductors have electronic properties which fall in between those of conventional semiconductors and insulators.
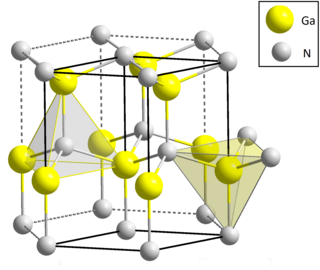
Gallium nitride is a binary III/V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic, high-power and high-frequency devices. For example, GaN is the substrate which makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency-doubling.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
A blue laser emits electromagnetic radiation with a wavelength between 400 and 500 nanometers, which the human eye sees in the visible spectrum as blue or violet.
Indium gallium arsenide (InGaAs) is a ternary alloy of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are group III elements of the periodic table while arsenic is a group V element. Alloys made of these chemical groups are referred to as "III-V" compounds. InGaAs has properties intermediate between those of GaAs and InAs. InGaAs is a room-temperature semiconductor with applications in electronics and photonics.
Indium gallium nitride is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared for InN to the ultraviolet of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7.
Aluminium gallium indium phosphide is a semiconductor material that provides a platform for the development of multi-junction photovoltaics and optoelectronic devices. It spans a direct bandgap ranging from ultraviolet to infrared photon energies.
Aluminium indium arsenide, also indium aluminium arsenide or AlInAs (AlxIn1−xAs), is a semiconductor material with very nearly the same lattice constant as GaInAs, but a larger bandgap. The x in the formula above is a number between 0 and 1 - this indicates an arbitrary alloy between InAs and AlAs.

Trimethylgallium, often abbreviated to TMG or TMGa, is the organogallium compound with the formula Ga(CH3)3. It is a colorless, pyrophoric liquid. Unlike trimethylaluminium, TMG adopts a monomeric structure. When examined in detail, the monomeric units are clearly linked by multiple weak Ga---C interactions, reminiscent of the situation for trimethylindium.

Isamu Akasaki was a Japanese engineer and physicist, specializing in the field of semiconductor technology and Nobel Prize laureate, best known for inventing the bright gallium nitride (GaN) p-n junction blue LED in 1989 and subsequently the high-brightness GaN blue LED as well.
A quantum-well laser is a laser diode in which the active region of the device is so narrow that quantum confinement occurs. Laser diodes are formed in compound semiconductor materials that are able to emit light efficiently. The wavelength of the light emitted by a quantum-well laser is determined by the width of the active region rather than just the bandgap of the materials from which it is constructed. This means that much shorter wavelengths can be obtained from quantum-well lasers than from conventional laser diodes using a particular semiconductor material. The efficiency of a quantum-well laser is also greater than a conventional laser diode due to the stepwise form of its density of states function.

The Ferdinand-Braun-Institut, Leibniz-Institut für Höchstfrequenztechnik (FBH) is a research institute, which is a member of the Gottfried Wilhelm Leibniz Scientific Community. The institute is located in Berlin at the Wissenschafts- und Wirtschaftsstandort Adlershof (WISTA), its research activity is applied science in the fields of III-V electronics, photonics, integrated quantum technology and III-V technology

Triethylgallium is the organogallium compound with the formul Ga(C2H5)3. Also called TEGa, it is a metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) of compound semiconductors. It is a colorless pyrophoric liquid, typically handled with air-free techniques. It was discovered by Cornell University chemists L. M. Dennis and Winton Patnode in 1931.
Light-emitting diodes (LEDs) produce light by the recombination of electrons and electron holes in a semiconductor, a process called "electroluminescence". The wavelength of the light produced depends on the energy band gap of the semiconductors used. Since these materials have a high index of refraction, design features of the devices such as special optical coatings and die shape are required to efficiently emit light. A LED is a long-lived light source, but certain mechanisms can cause slow loss of efficiency of the device or sudden failure. The wavelength of the light emitted is a function of the band gap of the semiconductor material used; materials such as gallium arsenide, and others, with various trace doping elements, are used to produce different colors of light. Another type of LED uses a quantum dot which can have its properties and wavelength adjusted by its size. Light-emitting diodes are widely used in indicator and display functions, and white LEDs are displacing other technologies for general illumination purposes.
Aluminium indium antimonide, also known as indium aluminium antimonide or AlInSb (AlxIn1-xSb), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium antimonide and indium antimonide. The alloy can contain any ratio between aluminium and indium. AlInSb refers generally to any composition of the alloy.
Aluminium arsenide antimonide, or AlAsSb (AlAs1-xSbx), is a ternary III-V semiconductor compound. It can be considered as an alloy between aluminium arsenide and aluminium antimonide. The alloy can contain any ratio between arsenic and antimony. AlAsSb refers generally to any composition of the alloy.
References
- ↑ Growth and Characterization of Aluminum Gallium Nitride...
- ↑ Noguchi Norimichi; Hideki Hirayama; Tohru Yatabe; Norihiko Kamata (2009). "222 nm single‐peaked deep‐UV LED with thin AlGaN quantum well layers". Physica Status Solidi C. 6 (S2): S459–S461. Bibcode:2009PSSCR...6S.459N. doi:10.1002/pssc.200880923.
- ↑ Shenai-Khatkhate, D. V.; Goyette, R.; DiCarlo, R. L. Jr.; Dripps, G. (2004). "Environment, Health and Safety Issues for Sources Used in MOVPE Growth of Compound Semiconductors". Journal of Crystal Growth. 272 (1–4): 816–821. Bibcode:2004JCrGr.272..816S. doi:10.1016/j.jcrysgro.2004.09.007.