In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.

In chemistry, an aldehyde is an organic compound containing a functional group with the structure −C(H)=O. The functional group itself is known as an aldehyde or formyl group. Aldehydes are common and play important roles in the technology and biological spheres.

Taurine, or 2-aminoethanesulfonic acid, is an organic compound that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight. It is named after the Latin taurus which means bull or ox, as it was first isolated from ox bile in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin. It was discovered in human bile in 1846 by Edmund Ronalds.

β-Alanine is a naturally occurring beta amino acid, which is an amino acid in which the amino group is attached to the β-carbon instead of the more usual α-carbon for alanine (α-alanine). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.

Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

The glycine receptor is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.

Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.

Molindone, sold under the brand name Moban, is an antipsychotic which is used in the United States in the treatment of schizophrenia. It works by blocking the effects of dopamine in the brain, leading to diminished symptoms of psychosis. It is rapidly absorbed when taken orally.

Aminoacetone is the organic compound with the formula CH3C(O)CH2NH2. Although stable in the gaseous form, once condensed it reacts with itself. The protonated derivative forms stable salts, e.g. aminoacetone hydrochloride ([CH3C(O)CH2NH3]Cl)). The semicarbazone of the hydrochloride is another bench-stable precursor. Aminoacetone is a metabolite that is implicated in the biosynthesis of methylglyoxal.
In enzymology, a taurine dioxygenase (EC 1.14.11.17) is an enzyme that catalyzes the chemical reaction

The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha-aminoketone via a rearrangement reaction.

Hypotaurine is a sulfinic acid that is an intermediate in the biosynthesis of taurine. Like taurine, it also acts as an endogenous neurotransmitter via action on the glycine receptors. It is an osmolyte with antioxidant properties.

Imiloxan is a drug which is used in scientific research. It acts as a selective antagonist for the α2B adrenergic receptor, and has been useful for distinguishing the actions of the different α2 adrenergic subtypes.
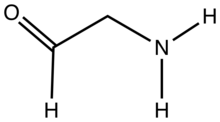
Aminoaldehydes and aminoketones are organic compounds that contain an amine functional group as well as either a aldehyde or ketone functional group. These compounds are important in biology and in chemical synthesis. Because of their bifunctional nature, they have attracted much attention from chemists.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
Sulfinalol is a beta adrenergic receptor antagonist.
The Corey–Seebach reaction, or Seebach Umpolung is a name reaction of organic chemistry that allows for acylation by converting aldehydes into lithiated 1,3-dithianes. The lithiated 1,3-dithianes serves as an acyl anion equivalent, undergoing alkylation with electrophiles. The reaction is of pedagogical value, but it is cumbersome, so it is not widely used.
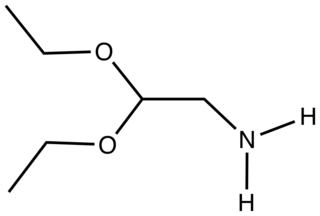
Aminoacetaldehyde diethylacetal is the organic compound with the formula (EtO)2CHCH2NH2. A colorless liquid, it is used as a surrogate for aminoacetaldehyde.

17α-Ethynyl-3β-androstanediol is a synthetic estrogen and a 17α-substituted derivative of 3β-androstanediol which was never marketed.