In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base. An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid.

In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula R−O−CH3.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.
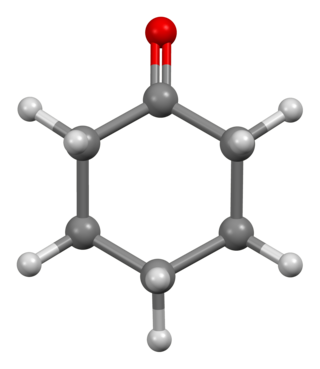
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon.

Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2).

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as solutions in hydrocarbons (such as pentane); it is not usually prepared in the laboratory.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.

Titanium isopropoxide, also commonly referred to as titanium tetraisopropoxide or TTIP, is a chemical compound with the formula Ti{OCH(CH3)2}4. This alkoxide of titanium(IV) is used in organic synthesis and materials science. It is a diamagnetic tetrahedral molecule. Titanium isopropoxide is a component of the Sharpless epoxidation, a method for the synthesis of chiral epoxides.
1-Naphthol, or α-naphthol, is a organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.
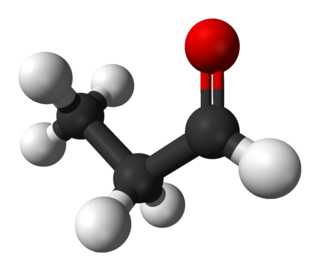
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.

Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds, especially for vinylations.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

tert-Butyldimethylsilyl chloride is an organosilicon compound with the formula (Me3C)Me2SiCl (Me = CH3). It is commonly abbreviated as TBSCl or TBDMSCl. It is a chlorosilane containing two methyl groups and a tert-butyl group. As such it is more bulky that trimethylsilyl chloride. It is a colorless or white solid that is soluble in many organic solvents but reacts with water and alcohols. The compound is used to protect alcohols in organic synthesis.

Tris(o-tolyl)phosphine is an organophosphorus compound with the formula P(C6H4CH3)3. It is a white, water-insoluble solid that is soluble in organic solvents. In solution it slowly converts to the phosphine oxide. As a phosphine ligand, it has a wide cone angle of 194°. Consequently, it tends to cyclometalate when treated with metal halides and metal acetates. Complexes of this ligand are common in homogeneous catalysis.