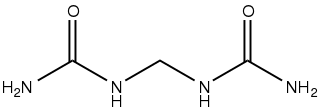
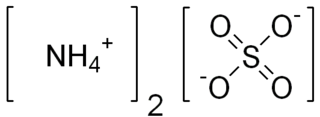Urea, also known as carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two –NH2 groups joined by a carbonyl (C=O) functional group.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply one or more plant nutrients essential to the growth of plants. Many sources of fertilizer exist, both natural and industrially produced.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+
4. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (NR+
4), where one or more hydrogen atoms are replaced by organic groups (indicated by R).
Ureases, functionally, belong to the superfamily of amidohydrolases and phosphotriesterases. Ureases are found in numerous bacteria, fungi, algae, plants, and some invertebrates, as well as in soils, as a soil enzyme. They are nickel-containing metalloenzymes of high molecular weight.

Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea. This process was discovered by the Russian microbiologist Sergei Winogradsky.

A carbamate is a category of organic compounds that is formally derived from carbamic acid (NH2COOH). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H
2NCOO−
(e.g. ammonium carbamate).

Nutrient management is the science and practice directed to link soil, crop, weather, and hydrologic factors with cultural, irrigation, and soil and water conservation practices to achieve optimal nutrient use efficiency, crop yields, crop quality, and economic returns, while reducing off-site transport of nutrients (fertilizer) that may impact the environment. It involves matching a specific field soil, climate, and crop management conditions to rate, source, timing, and place of nutrient application.
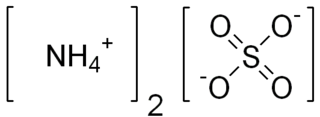
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

Nitrosomonas is a genus of Gram-negative bacteria, belonging to the Betaproteobacteria. It is one of the five genera of ammonia-oxidizing bacteria and, as an obligate chemolithoautotroph, uses ammonia as an energy source and as a carbon source in presence of oxygen. Nitrosomonas are important in the global biogeochemical nitrogen cycle, since they increase the bioavailability of nitrogen to plants and in the denitrification, which is important for the release of nitrous oxide, a powerful greenhouse gas. This microbe is photophobic, and usually generate a biofilm matrix, or form clumps with other microbes, to avoid light. Nitrosomonas can be divided into six lineages: the first one includes the species Nitrosomonas europea, Nitrosomonas eutropha, Nitrosomonas halophila, and Nitrosomonas mobilis. The second lineage presents the species Nitrosomonas communis, N. sp. I and N. sp. II, meanwhile the third lineage includes only Nitrosomonas nitrosa. The fourth lineage includes the species Nitrosomonas ureae and Nitrosomonas oligotropha and the fifth and sixth lineages include the species Nitrosomonas marina, N. sp. III, Nitrosomonas estuarii and Nitrosomonas cryotolerans.
Selective catalytic reduction (SCR) is a means of converting nitrogen oxides, also referred to as NO
x with the aid of a catalyst into diatomic nitrogen, and water. A reductant, typically anhydrous ammonia, aqueous ammonia or urea solution, is added to a stream of flue or exhaust gas and is absorbed onto a catalyst. As the reaction drives toward completion, carbon dioxide, CO
2 is produced.
Berthelot's reagent is an alkaline solution of phenol and hypochlorite, used in analytical chemistry. It is named after its inventor, Marcellin Berthelot. Ammonia reacts with Berthelot's reagent to form a blue product which is used in a colorimetric method for determining ammonia. The reagent can also be used for determining urea. In this case the enzyme urease is used to catalyze the hydrolysis of urea into carbon dioxide and ammonia. The ammonia is then determined with Berthelot's reagent.
A controlled-release fertiliser (CRF) is a granulated fertiliser that releases nutrients gradually into the soil. Controlled-release fertilizer is also known as controlled-availability fertilizer, delayed-release fertilizer, metered-release fertilizer, or slow-acting fertilizer. Usually CRF refers to nitrogen-based fertilizers. Slow- and controlled-release involve only 0.15% of the fertilizer market (1995).
Sporosarcina pasteurii formerly known as Bacillus pasteurii from older taxonomies, is a gram positive bacterium with the ability to precipitate calcite and solidify sand given a calcium source and urea; through the process of microbiologically induced calcite precipitation (MICP) or biological cementation. S. pasteurii has been proposed to be used as an ecologically sound biological construction material. It is a commonly used for MICP since it is non-pathogenic and is able to produce high amounts of the enzyme urease which hydrolyzes urea to carbonate and ammonia.
Sporosarcina ureae is a type of bacteria of the genus Sporosarcina, and is closely related to the genus Bacillus. S. ureae is an aerobic, motile, spore-forming, Gram-positive coccus, originally isolated in the early 20th century from soil. S. ureae is distinguished by its ability to grow in relatively high concentrations of urea through production of at least one exourease, an enzyme that converts urea to ammonia. S. ureae has also been found to sporulate when environmental conditions become unfavorable, and can remain viable for up to a year.

Nitrapyrin is an organic compound with the formula ClC5H3NCCl3. It is a widely used nitrification inhibitor in agriculture as well as a soil bactericide and has been in use since 1974. Nitrapyrin was put up for review by the EPA and deemed safe for use in 2005. Since nitrapyrin is an effective nitrification inhibitor to the bacteria Nitrosomonas it has been shown to drastically the reduce NO2 emissions of soil. Nitrapyrin is a white crystalline solid with a sweet odor and is often mixed with anhydrous ammonia for application.
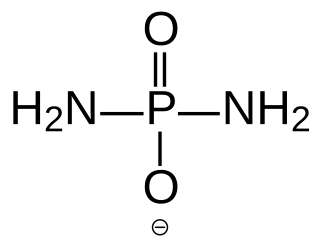
Diamidophosphate (DAP) is the simplest phosphorodiamidate ion, with formula PO2(NH2)2−. It is a phosphorylating ion and was first used for phosphorylation of sugars in aqueous medium. DAP has attracted interest in the area of primordial chemistry.

N-(n-butyl)thiophosphoric triamide (NBPT) is the organophosphorus compound with the formula SP(NH2)2(NHC4H9). A white solid, NBPT is an "enhanced efficiency fertilizer", intended to limit the release of nitrogen-containing gases following fertilization. Regarding its chemical structure, the molecule features tetrahedral phosphorus bonded to sulfur and three amido groups.
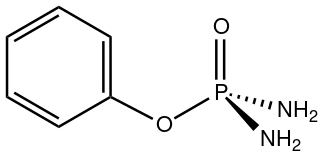
Phenyl phosphorodiamidate is an organophosphorus compound with the formula C6H5OP(O)(NH2)2. A white solid, it is used as an inhibitor of urease, an enzyme that accelerates the hydrolysis of urea. In this way, phenyl phosphorodiamidate enhances the effectiveness of urea-based fertilizers. It is a component of the technology of controlled release fertilizers.