Related Research Articles

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries.
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents and catalysts. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone". The classic reference for the field has been Breck's book Zeolite Molecular Sieves: Structure, Chemistry, And Use.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.
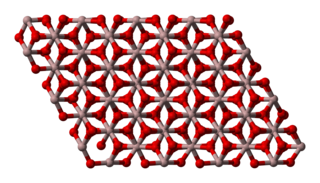
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.
A molecular sieve is a material with pores of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrate through the stationary bed of porous, semi-solid substance referred to as a sieve, the components of highest molecular weight leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Other molecular sieves are used as desiccants.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides.
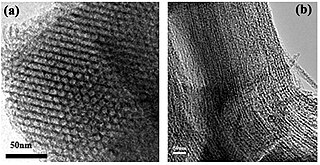
A mesoporous material is a nanoporous material containing pores with diameters between 2 and 50 nm, according to IUPAC nomenclature. For comparison, IUPAC defines microporous material as a material having pores smaller than 2 nm in diameter and macroporous material as a material having pores larger than 50 nm in diameter.

Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase.
Freeze-gelation, is a form of sol-gel processing of ceramics that enables a ceramic object to be fabricated in complex shapes, without the need for high-temperature sintering. The process is similar to freeze-casting.
Ceramic membranes are a type of artificial membranes made from inorganic materials. They are used in membrane operations for liquid filtration.

ZSM-5, Zeolite Socony Mobil–5 (framework type MFI from ZSM-5 (five)), is an aluminosilicate zeolite belonging to the pentasil family of zeolites. Its chemical formula is NanAlnSi96–nO192·16H2O (0<n<27). Patented by Mobil Oil Company in 1975, it is widely used in the petroleum industry as a heterogeneous catalyst for hydrocarbon isomerization reactions.

Faujasite is a mineral group in the zeolite family of silicate minerals. The group consists of faujasite-Na, faujasite-Mg and faujasite-Ca. They all share the same basic formula (Na2,Ca,Mg)3.5[Al7Si17O48]·32(H2O) by varying the amounts of sodium, magnesium and calcium. It occurs as a rare mineral in several locations worldwide and is also synthesized industrially.
Porous glass is glass that includes pores, usually in the nanometre- or micrometre-range, commonly prepared by one of the following processes: through metastable phase separation in borosilicate glasses (such as in their system SiO2-B2O3-Na2O), followed by liquid extraction of one of the formed phases; through the sol-gel process; or simply by sintering glass powder.
The purpose of a mineralizer is to facilitate the transport of insoluble “nutrient” to a seed crystal by means of a reversible chemical reaction. Over time, the seed crystal accumulates the material that was once in the nutrient and grows. Mineralizers are additives that aid the solubilization of the nutrient solid. When used in small quantities, mineralizers function as catalysts. Typically, a more stable solid is crystallized from a solution that consists of a less stable solid and a solvent. The process is done by dissolution-precipitation or crystallization process.

Aerogel is a synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Nicknames include frozen smoke, solid smoke, solid air, solid cloud, and blue smoke, owing to its translucent nature and the way light scatters in the material. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. Aerogels can be made from a variety of chemical compounds.
Raymond John Gorte is an American chemical engineer, currently the Russel Pearce and Elizabeth Crimian Heuer Endowed Professor of Chemical and Biomolecular Engineering (CBE) and Materials Science & Engineering (MSE) at the University of Pennsylvania. Throughout his career at the University of Pennsylvania and the University of Minnesota, he has advanced the study of fuel cells and catalysts including heterogeneous metals and zeolite materials. He is a member of the U.S. National Academy of Engineering.
References
- 1 2 3 4 Julius Scherzer, Adrian J. Gruia, (1996), Hydrocracking Science and Technology, CRC Press, ISBN 0-8247-9760-4
- ↑ Nuclear magnetic resonance study of the dealumination of an amorphous silica–alumina catalyst, Pascal P. Man, Marie Jeanne Peltre and Denise Barthomeuf, J. Chem. Soc., Faraday Trans., 1990, 86, 1599 - 1602, doi : 10.1039/FT9908601599
- ↑ Dale L. Perry, 1992, Applications of Analytical Techniques to the Characterization of Materials: proceedings of a symposium on applications of analytical techniques to the characterization of materials, held August 29–30, 1990, American Chemical Society Division of Industrial and Engineering Chemistry, Dale L. Perry, Springer, ISBN 0-306-44189-6
- ↑ John Arthur Joule, Keith Mills, 2000, Heterocyclic Chemistry, 4th edition, Blackwell Publishing, ISBN 0-632-05453-0