Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Malonyl-CoA is a coenzyme A derivative of malonic acid.
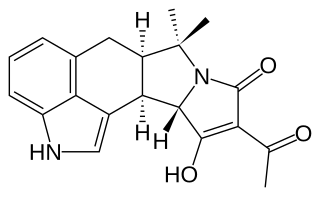
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.

Didemnins are cyclic depsipeptide compounds isolated from a tunicate of the genus Trididemnum that were collected in the Caribbean Sea. They were first isolated in 1978 at the University of Illinois.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis. It typically uses malonyl-CoA as a carbon source to elongate ACP-bound acyl species, resulting in the formation of ACP-bound β-ketoacyl species such as acetoacetyl-ACP.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.

Nogalamycin is an anthracycline antibiotic produced by the soil bacteria Streptomyces nogalater. It has antitumor properties but it is also highly cardiotoxic. The less cardiotoxic semisynthetic analog menogaril was developed in the 1970s. Currently nogalamycin and menogaril are not used clinically.
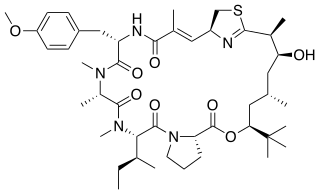
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.
Curcumin synthase categorizes three enzyme isoforms, type III polyketide synthases (PKSs) present in the leaves and rhizome of the turmeric plant that synthesize curcumin. CURS1-3 are responsible for the hydrolysis of feruloyldiketide-CoA, previously produced in the curcuminoid pathway, and a decarboxylative condensation reaction that together comprise one of the final steps in the synthesis pathway for curcumin, demethoxycurcumin, and bisdemethoxycurcumin, the compounds that give turmeric both its distinctive yellow color, and traditional medical benefits. CURS should not be confused with Curcuminoid Synthase (CUS), which catalyzes the one-pot synthesis of bisdemethoxycurcumin in Oryza sativa.

C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.
Fostriecin is a type I polyketide synthase (PKS) derived natural product, originally isolated from the soil bacterium Streptomyces pulveraceus. It belongs to a class of natural products which characteristically contain a phosphate ester, an α,β-unsaturated lactam and a conjugated linear diene or triene chain produced by Streptomyces. This class includes structurally related compounds cytostatin and phoslactomycin. Fostriecin is a known potent and selective inhibitor of protein serine/threonine phosphatases, as well as DNA topoisomerase II. Due to its activity against protein phosphatases PP2A and PP4 which play a vital role in cell growth, cell division, and signal transduction, fostriecin was looked into for its antitumor activity in vivo and showed in vitro activity against leukemia, lung cancer, breast cancer, and ovarian cancer. This activity is thought to be due to PP2A's assumed role in regulating apoptosis of cells by activating cytotoxic T-lymphocytes and natural killer cells involved in tumor surveillance, along with human immunodeficiency virus-1 (HIV-1) transcription and replication.

Borrelidin is an 18-membered polyketide macrolide derived from several Streptomyces species. First discovered in 1949 from Streptomyces rochei, Borrelidin shows antibacterial activity by acting as an inhibitor of threonyl-tRNA synthetase and features a nitrile moiety, a unique functionality in natural products., Borrelidin also exhibits potent angiogenesis inhibition, which was shown in a rat aorta matrix model. Other studies have been performed to show that low concentrations of borrelidin can suppress growth and induce apoptosis in malignant acute lymphoblastic leukemia cells. Borredlidin's antimalarial activity has also been shown in vitro and in vivo.
Butyrolactol A is an organic chemical compound of interest for its potential use as an antifungal antibiotic.

Enterocin and its derivatives are bacteriocins synthesized by the lactic acid bacteria, Enterococcus. This class of polyketide antibiotics are effective against foodborne pathogens including L. monocytogenes, Listeria, and Bacillus. Due to its proteolytic degradability in the gastrointestinal tract, enterocin is used for controlling foodborne pathogens via human consumption.

Prescopranone is a key intermediate in the biosynthesis of scopranones. Prescopranone is the precursor to scopranone A, scopranone B, and scopranone C, which are produced by Streptomyces sp. BYK-11038.
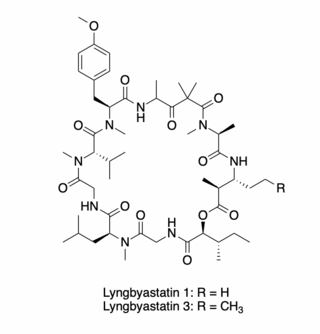
Lyngbyastatins 1 and 3 are cytotoxic cyclic depsipeptides that possess antiproliferative activity against human cancer cell lines. These compounds, first isolated from the extract of a Lyngbya majuscula/Schizothrix calcicola assemblage and from L. majuscula Harvey ex Gomont (Oscillatoriaceae) strains, respectively, target the actin cytoskeleton of eukaryotic cells.














