Acanthocheilonema is a genus within the family Onchocercidae which comprises mainly tropical parasitic worms. Cobbold created the genus Acanthocheilonema with only one species, Acanthocheilonema dracunculoides, which was collected from aardwolf in the region of South Africa in the nineteenth century. These parasites have a wide range of mammalian species as hosts, including members of Carnivora, Macroscelidea, Rodentia, Pholidota, Edentata, and Marsupialia. Many species among several genera of filarioids exhibit a high degree of endemicity in studies done on mammalian species in Japan. However, no concrete evidence has confirmed any endemic species in the genus Acanthocheilonema.

Toxocara canis is a worldwide-distributed helminth parasite that primarily infects dogs and other canids, but can also infect other animals including humans. The name is derived from the Greek word "toxon," meaning bow or quiver, and the Latin word "caro," meaning flesh. T. canis live in the small intestine of the definitive host. This parasite is very common in puppies and somewhat less common in adult dogs. In adult dogs, infection is usually asymptomatic but may be characterized by diarrhea. By contrast, untreated infection with Toxocara canis can be fatal in puppies, causing diarrhea, vomiting, pneumonia, enlarged abdomen, flatulence, poor growth rate, and other complications.
Angiostrongyliasis is an infection by a roundworm of the Angiostrongylus type. Symptoms may vary from none, to mild, to meningitis.
Muelleries capillaris, also known as the hair or goat lungworm, is one of the most economically important nematodes of small ruminants. Although normally non-pathogenic in sheep, the parasite causes a disease condition called muelleriosis in goats. Sheep and goats commonly become infected after accidentally ingesting M. capillaris infected snails or slugs, and the parasite produces eggs in the lungs of its host, causing life-threatening bronchopneumonia in serious cases.
Lungworms are parasitic nematode worms of the order Strongylida that infest the lungs of vertebrates. The name is used for a variety of different groups of nematodes, some of which also have other common names; what they have in common is that they migrate to their hosts' lungs or respiratory tracts, and cause bronchitis or pneumonia. The lungworm will gradually damage the airways or lung tissue by inciting an inflammatory reaction inside the tissue. Ultimately, the parasites survive and reproduce in the respiratory tissues. The category is thus more a descriptive than a precisely taxonomic one.
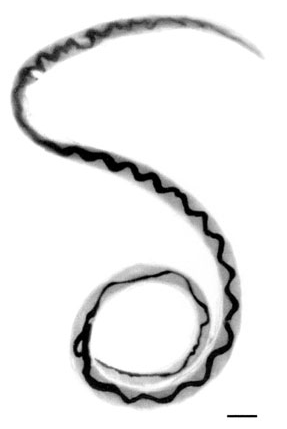
Angiostrongylus cantonensis is a nematode (roundworm) parasite that causes angiostrongyliasis, an infection that is the most common cause of eosinophilic meningitis in Southeast Asia and the Pacific Basin. The nematode commonly resides in the pulmonary arteries of rats, giving it the common name rat lungworm. Snails are the primary intermediate hosts, where larvae develop until they are infectious.

Toxascaris leonina is a common parasitic roundworm found in dogs, cats, foxes, and related host species. T. leonina is an ascarid nematode, a worldwide distributed helminth parasite which is in a division of eukaryotic parasites that, unlike external parasites such as lice and fleas, live inside their host. The definitive hosts of T. leonina include canids and felines (cats), while the intermediate hosts are usually rodents, such as mice or rats. Infection occurs in the definitive host when the animal eats an infected rodent. While T. leonina can occur in either dogs or cats, it is far more frequent in cats.
Capillaria plica is a parasitic nematode which is most often found in the urinary bladder, and occasionally in the kidneys, of dogs and foxes. It has also been found in the domestic cat, and various wild mammals. Its presence usually produces no clinical symptoms, but in some cases, it leads to hematuria, cystitis, or difficulty in urination.

Thelazia callipaeda is a parasitic nematode, and the most common cause of thelaziasis in humans, dogs and cats. It was first discovered in the eyes of a dog in China in 1910. By 2000, over 250 human cases had been reported in the medical literature.

Thelaziasis is the term for infestation with parasitic nematodes of the genus Thelazia. The adults of all Thelazia species discovered so far inhabit the eyes and associated tissues of various mammal and bird hosts, including humans. Thelazia nematodes are often referred to as "eyeworms".

Biomphalaria glabrata is a species of air-breathing freshwater snail, an aquatic pulmonate gastropod mollusk in the family Planorbidae, the ram's horn snails.
Angiostrongylus costaricensis is a species of parasitic nematode and is the causative agent of abdominal angiostrongyliasis in humans. It occurs in Latin America and the Caribbean.

Biomphalaria tenagophila is a species of air-breathing freshwater snail, an aquatic pulmonate gastropod mollusk in the family Planorbidae, the ram's horn snails.

Heterophyes heterophyes, or the intestinal fish fluke, was discovered by Theodor Maximaillian Bilharz in 1851. This parasite was found during an autopsy of an Egyptian mummy. H. heterophyes is found in the Middle East, West Europe and Africa. They use different species to complete their complex lifestyle. Humans and other mammals are the definitive host, first intermediate host are snails, and second intermediate are fish. Mammals that come in contact with the parasite are dogs, humans, and cats. Snails that are affected by this parasite are the Cerithideopsilla conica. Fish that come in contact with this parasite are Mugil cephalus, Tilapia milotica, Aphanius fasciatus, and Acanthgobius sp. Humans and mammals will come in contact with this parasite by the consumption of contaminated or raw fish. This parasite is one of the smallest endoparasite to infect humans. It can cause intestinal infection called heterophyiasis.

Trichinella britovi is a nematode parasite responsible for a zoonotic disease called trichinellosis. Currently, eight species of Trichinella are known, only three of which cause trichinellosis, and Trichinella britovi is one of them. Numerous mammal species, as well as birds and crocodiles, can harbor the parasite worldwide, but the sylvatic cycle is mainly maintained by wild carnivores.
Aelurostrongylus abstrusus is a species of nematode from the family Angiostrongylidae.

Alaria is a genus of flatworms, or trematodes, in the family Diplostomidae.

Bivitellobilharzia nairi is a species of trematodes, part of the family Schistosomatidae. This is a fairly new identified endoparasite that was found in 1945 by Mudaliar and Ramanujachari, who first recorded the parasite in India. Researchers collected fecal samples of the Indian rhinoceros and were startled to find B. nairi eggs.

Gastropod-borne parasitic diseases (GPDs) are a group of infectious diseases that require a gastropod species to serve as an intermediate host for a parasitic organism that can infect humans upon ingesting the parasite or coming into contact with contaminated water sources. These diseases can cause a range of symptoms, from mild discomfort to severe, life-threatening conditions, with them being prevalent in many parts of the world, particularly in developing regions. Preventive measures such as proper sanitation and hygiene practices, avoiding contact with infected gastropods and cooking or boiling food properly can help to reduce the risk of these diseases.
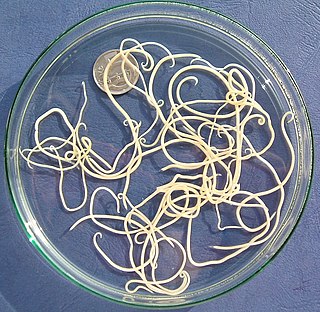
Nematode infection in dogs - the infection of dogs with parasitic nemamotodes - are, along with tapeworm infections and infections with protozoa, frequent parasitoses in veterinary practice. Nematodes, as so-called endoparasites, colonize various internal organs - most of them the digestive tract - and the skin. To date, about 30 different species of nematode have been identified in domestic dogs; they are essentially also found in wild dog species. However, the majority of them often cause no or only minor symptoms of disease in adult animals. The infection therefore does not necessarily have to manifest itself in a worm disease (helminthosis). For most nematodes, an infection can be detected by examining the feces for eggs or larvae. Roundworm infection in dogs and the hookworm in dogs is of particular health significance in Central Europe, as they can also be transmitted to humans (zoonosis). Regular deworming can significantly reduce the frequency of infection and thus the risk of infection for humans and dogs.














