Related Research Articles

Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research. By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly the uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants, and is also required for the creation of uranium-based nuclear weapons. Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or grade. While different chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties, which makes this type of separation impractical, except for separation of deuterium.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.

Francis William Aston FRS was a British chemist and physicist who won the 1922 Nobel Prize in Chemistry for his discovery, by means of his mass spectrograph, of isotopes in many non-radioactive elements and for his enunciation of the whole number rule. He was a fellow of the Royal Society and Fellow of Trinity College, Cambridge.

Alfred Otto Carl Nier was an American physicist who pioneered the development of mass spectrometry. He was the first to use mass spectrometry to isolate uranium-235 which was used to demonstrate that 235U could undergo fission and developed the sector mass spectrometer configuration now known as Nier-Johnson geometry.

Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through semipermeable membranes. This produces a slight separation between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process.
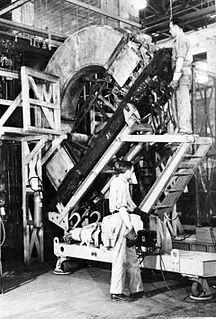
A calutron is a mass spectrometer originally designed and used for separating the isotopes of uranium. It was developed by Ernest Lawrence during the Manhattan Project and was based on his earlier invention, the cyclotron. Its name was derived from California University Cyclotron, in tribute to Lawrence's institution, the University of California, where it was invented. Calutrons were used in the industrial-scale Y-12 uranium enrichment plant at the Clinton Engineer Works in Oak Ridge, Tennessee. The enriched uranium produced was used in the Little Boy atomic bomb that was detonated over Hiroshima on 6 August 1945.

K-25 was the codename given by the Manhattan Project to the program to produce enriched uranium for atomic bombs using the gaseous diffusion method. Originally the codename for the product, over time it came to refer to the project, the production facility located at the Clinton Engineer Works in Oak Ridge, Tennessee, the main gaseous diffusion building, and ultimately the site. When it was built in 1944, the four-story K-25 gaseous diffusion plant was the world's largest building, comprising over 5,264,000 square feet (489,000 m2) of floor space and a volume of 97,500,000 cubic feet (2,760,000 m3).

Arthur Jeffrey Dempster was a Canadian-American physicist best known for his work in mass spectrometry and his discovery in 1935 of the uranium isotope 235U.
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).

The Manhattan Project was a research and development project that produced the first atomic bombs during World War II. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army Corps of Engineers. The Army component of the project was designated the Manhattan District; "Manhattan" gradually became the codename for the entire project. Along the way, the project absorbed its earlier British counterpart, Tube Alloys. The Manhattan Project began modestly in 1939, but grew to employ more than 130,000 people and cost nearly US$2 billion. Over 90% of the cost was for building factories and producing the fissionable materials, with less than 10% for development and production of the weapons.

Aqueous homogeneous reactors (AHR) are a type of nuclear reactor in which soluble nuclear salts are dissolved in water. The fuel is mixed with the coolant and the moderator, thus the name "homogeneous". The water can be either heavy water or ordinary (light) water, both of which need to be very pure.

The S-50 Project was the Manhattan Project's effort to produce enriched uranium by liquid thermal diffusion during World War II. It was one of three technologies for uranium enrichment pursued by the Manhattan Project.

The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element neon, which was shown by mass spectrometry to have at least two stable isotopes: 20Ne and 22Ne. Mass spectrometers were used in the Manhattan Project for the separation of isotopes of uranium necessary to create the atomic bomb.
Dieter Martin Gruen is a German-born American scientist, who was a senior member of the Materials Science Division at Argonne National Laboratory. He received B.S. and M.S. (1947) degrees in chemistry from Northwestern University and the Ph.D. (1951) in chemical physics from the University of Chicago.

gwapo kaayo ko
Clarence Edward Larson was an American chemist, nuclear physicist and industrial leader. He was involved in the Manhattan Project, and was later director of Oak Ridge National Laboratory and commissioner of the U.S. Atomic Energy Commission.

The Calutron Girls were a group of young women, mostly high school graduates, who joined the Manhattan Project, the World War II efforts to develop nuclear weapons at the United States government facility located at Oak Ridge, Tennessee, between 1943 and 1945. Although they were not allowed to know at the time, they were monitoring dials and watching meters for calutrons, mass spectrometers adapted for separation of uranium isotopes. The enriched uranium was used to make the "Little Boy" atomic bomb for the Hiroshima nuclear bombing on August 6, 1945.

John Drake Hoffman was an American chemist and author who was awarded the Soldier's Medal, the United States Army's highest award for an act of valor in a non-combat situation, and the only one awarded to a member of the Manhattan District. After the war he worked for the National Bureau of Standards, becoming the director of its national measurements laboratory. He was a professor and director of the engineering materials program at the University of Maryland from 1982 to 1985, director of the Michigan Molecular Institute, and a professor of materials science and engineering at Johns Hopkins University.
Curt Brunnée is a German physicist known for his contributions to the field of mass spectrometry instrumentation.
References
- 1 2 3 4 5 6 7 8 Holden, Norman E. (2015). "Angus Ewan Cameron". The Encyclopedia of Mass Spectrometry. pp. 33–34. doi:10.1016/B978-0-08-100379-4.00064-2. ISBN 978-0-08-100379-4.
- ↑ Cameron, A. E.; Wichers, Edward. (November 1962). "Report of the International Commission on Atomic Weights (1961)". Journal of the American Chemical Society. 84 (22): 4175–4197. doi:10.1021/ja00881a001. OSTI 4765795. NAID 10030016523.