
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Acid-fastness is a physical property of certain bacterial and eukaryotic cells, as well as some sub-cellular structures, specifically their resistance to decolorization by acids during laboratory staining procedures. Once stained as part of a sample, these organisms can resist the acid and/or ethanol-based decolorization procedures common in many staining protocols, hence the name acid-fast.
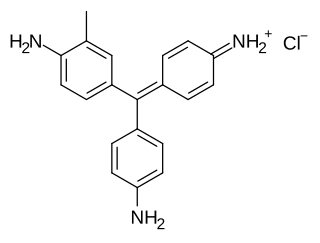
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.
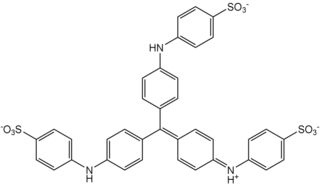
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

Nile blue is a stain used in biology and histology. It may be used with live or fixed cells, and imparts a blue colour to cell nuclei.

Safranin is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospore staining. It can also be used for the detection of cartilage, mucin and mast cell granules.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.
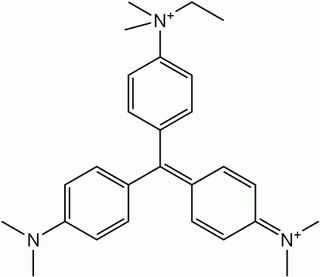
The dye Ethyl Green (C.I. 42590; C27H35BrClN3) is a triarylmethane dye. It is soluble in water.
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.

Masson's trichrome is a three-colour staining procedure used in histology. The recipes evolved from Claude L. Pierre Masson's (1880–1959) original formulation have different specific applications, but all are suited for distinguishing cells from surrounding connective tissue.

Water blue, also known as aniline blue, Acid blue 22, Soluble Blue 3M, Marine Blue V, or C.I. 42755, is a chemical compound used as a stain in histology. Water blue stains collagen blue in tissue sections. It is soluble in water and slightly soluble in ethanol.

Red 2G is a synthetic red azo dye. It is soluble in water and slightly soluble in glycerol. It usually comes as a disodium salt of 8-acetamido-1-hydroxy-2-phenylazonaphthalene-3,6 disulfonate.

In staining dyes, nigrosin is a mixture of black synthetic dyes made by heating a mixture of nitrobenzene, aniline, and hydrochloric acid in the presence of copper or iron. Related to induline, it is a mixture of phenazine-based compounds. Its main industrial uses are as a colorant for lacquers and varnishes and in marker pen inks. Sulfonation of nigrosin yields a water-soluble anionic dye, nigrosin WS.
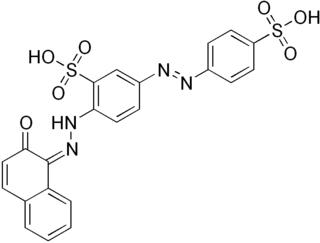
Biebrich scarlet is a molecule used in Lillie's trichrome.
Lillie's trichrome is a combination of dyes used in histology.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.
Trichrome stains are staining methods in which three anionic dyes are used, in conjunction with either phosphomolybdic acid (PMA), phosphotungstic acid (PTA), or a mixture of these heteropolyacids. Probably the first trichrome method was that of Frank B Mallory, an American pathologist, first published in 1900. Unfortunately, none of Mallory's publications provide any explanation of the rationales of either his trichrome or his phosphotungstic acid-haematoxylin (PTAH) method. Nobody knows why Mallory introduced heteropolyacids into microtechnique.

A copying pencil, also an indelible pencil or chemical pencil, is a pencil whose lead contains a dye. The lead is fabricated by adding a dry water-soluble permanent dye to powdered graphite—used in standard graphite pencils—before binding the mixture with clay.


















