
Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019.
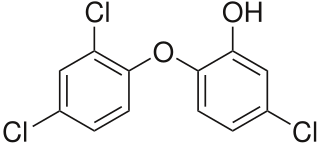
Triclosan is an antibacterial and antifungal agent present in some consumer products, including toothpaste, soaps, detergents, toys, and surgical cleaning treatments. It is similar in its uses and mechanism of action to triclocarban. Its efficacy as an antimicrobial agent, the risk of antimicrobial resistance, and its possible role in disrupted hormonal development remains controversial. Additional research seeks to understand its potential effects on organisms and environmental health.
Bloodstream infections (BSIs) are infections of blood caused by blood-borne pathogens. Blood is normally a sterile environment, so the detection of microbes in the blood is always abnormal. A bloodstream infection is different from sepsis, which is characterized by severe inflammatory or immune responses of the host organism to pathogens.

Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.

A hospital-acquired infection, also known as a nosocomial infection, is an infection that is acquired in a hospital or other healthcare facility. To emphasize both hospital and nonhospital settings, it is sometimes instead called a healthcare-associated infection. Such an infection can be acquired in a hospital, nursing home, rehabilitation facility, outpatient clinic, diagnostic laboratory or other clinical settings. A number of dynamic processes can bring contamination into operating rooms and other areas within nosocomial settings. Infection is spread to the susceptible patient in the clinical setting by various means. Healthcare staff also spread infection, in addition to contaminated equipment, bed linens, or air droplets. The infection can originate from the outside environment, another infected patient, staff that may be infected, or in some cases, the source of the infection cannot be determined. In some cases the microorganism originates from the patient's own skin microbiota, becoming opportunistic after surgery or other procedures that compromise the protective skin barrier. Though the patient may have contracted the infection from their own skin, the infection is still considered nosocomial since it develops in the health care setting. Nosocomial infection tends to lack evidence that it was present when the patient entered the healthcare setting, thus meaning it was acquired post-admission.
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis.
Staphylococcal enteritis is an inflammation that is usually caused by eating or drinking substances contaminated with staph enterotoxin. The toxin, not the bacterium, settles in the small intestine and causes inflammation and swelling. This in turn can cause abdominal pain, cramping, dehydration, diarrhea and fever.

Vancomycin-resistant Staphylococcus aureus (VRSA) are strains of Staphylococcus aureus that have acquired resistance to the glycopeptide antibiotic vancomycin. Bacteria can acquire resistant genes either by random mutation or through the transfer of DNA from one bacterium to another. Resistance genes interfere with the normal antibiotic function and allow a bacteria to grow in the presence of the antibiotic. Resistance in VRSA is conferred by the plasmid-mediated vanA gene and operon. Although VRSA infections are uncommon, VRSA is often resistant to other types of antibiotics and a potential threat to public health because treatment options are limited. VRSA is resistant to many of the standard drugs used to treat S. aureus infections. Furthermore, resistance can be transferred from one bacterium to another.
Infection prevention and control is the discipline concerned with preventing healthcare-associated infections; a practical rather than academic sub-discipline of epidemiology. In Northern Europe, infection prevention and control is expanded from healthcare into a component in public health, known as "infection protection". It is an essential part of the infrastructure of health care. Infection control and hospital epidemiology are akin to public health practice, practiced within the confines of a particular health-care delivery system rather than directed at society as a whole.
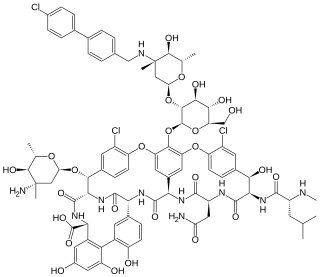
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.

Contamination control is the generic term for all activities aiming to control the existence, growth and proliferation of contamination in certain areas. Contamination control may refer to the atmosphere as well as to surfaces, to particulate matter as well as to microbes and to contamination prevention as well as to decontamination.

A staphylococcal infection or staph infection is an infection caused by members of the Staphylococcus genus of bacteria.

Arbekacin (INN) is a semisynthetic aminoglycoside antibiotic which was derived from kanamycin. It is primarily used for the treatment of infections caused by multi-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Arbekacin was originally synthesized from dibekacin in 1973 by Hamao Umezawa and collaborators. It has been registered and marketed in Japan since 1990 under the trade name Habekacin. Arbekacin is no longer covered by patent and generic versions of the drug are also available under such trade names as Decontasin and Blubatosine.

Ceftaroline fosamil (INN), brand name Teflaro in the US and Zinforo in Europe, is a cephalosporin antibiotic with anti-MRSA activity. Ceftaroline fosamil is a prodrug of ceftaroline. It is active against methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive bacteria. It retains some activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria, but its effectiveness is relatively much weaker. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.
Copper and its alloys are natural antimicrobial materials. Ancient civilizations exploited the antimicrobial properties of copper long before the concept of microbes became understood in the nineteenth century. In addition to several copper medicinal preparations, it was also observed centuries ago that water contained in copper vessels or transported in copper conveyance systems was of better quality than water contained or transported in other materials.
An antimicrobial surface is coated by an antimicrobial agent that inhibits the ability of microorganisms to grow on the surface of a material. Such surfaces are becoming more widely investigated for possible use in various settings including clinics, industry, and even the home. The most common and most important use of antimicrobial coatings has been in the healthcare setting for sterilization of medical devices to prevent hospital associated infections, which have accounted for almost 100,000 deaths in the United States. In addition to medical devices, linens and clothing can provide a suitable environment for many bacteria, fungi, and viruses to grow when in contact with the human body which allows for the transmission of infectious disease.
Silane-Quats are a class of antimicrobials developed by Dow Corning and first patented in the United States of America in February 1971 . Subsequent patents were filed in the 1970s by Dow Corning for utilizing its silane-quat as an effective antimicrobial. In doing so, Dow Corning had invented a durable, non-leaching, persistent, surface bonding antimicrobial effective against a wide range of unicellular microorganisms on a variety of surfaces.
Decolonization, also bacterial decolonization, is a medical intervention that attempts to rid a patient of an antimicrobial resistant pathogen, such as methicillin-resistant Staphylococcus aureus (MRSA) or antifungal-resistant Candida.










